#NephJC chat Tuesday March 29th 9 pm Eastern
Wednesday March 30th 8 pm BST, 12 noon Pacific
N Engl J Med. 1998 Aug 27;339(9):584-90.
The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin.
Besarab A1, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin D
PMID: 9718337 Free Full text available at NEJM
AND
Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-1839. [Epub ahead of print]
The Effect of Erythropoietin-Stimulating Agents on Health-Related Quality of Life in Anemia of Chronic Kidney Disease: A Systematic Review and Meta-analysis.
Collister D, Komenda P, Hiebert B, Gunasekara R, Xu Y, Eng F, Lerner B, Macdonald K, Rigatto C, Tangri N.
PMID: 26881842
This topic has been reviewed as a Misstep in Nephrology in the ongoing #NephMadness. See the summary by Joel Topf. There is also a typically acerbic commentary from Vinay Prasad, author of Ending Medical Reversals, here. In this chat, we will focus a bit more narrowly on the reporting in the original Normal hematocrit trial, and the recent systematic review in Annals, which examined the effect of Epo on quality of life (QoL).
Update [April 4th 2016]: In something of a coup, the #NephMadness team have published a commentary from Daniel Coyne, online yesterday. Read it here.
Pre-Chat Summary
One of the earliest erythropoietin trials was conducted by Canadians, the Canadian Erthyropoietin Study group. In a small placebo controlled trial, they demonstrated that using erythropoietin to keep hemoglobins higher (72 g/L in placebo group compared to 102 and 117 in the two treatment groups) improved physical functioning. Erythropoietin also caused a higher blood pressure, and greater vascular access failures, a harbinger of data to follow.
The Normal Hematocrit Trial
This was a landmark trial, and unsurprisingly, published in the NEJM, and has been cited almost 900 times. Funded by Amgen, the trial randomized 1,233 hemodialysis patients with heart disease (congestive heart failure or ischemic heart disease) to one of two hematocrits, 30% versus 42% (the latter being the 'normal' hematocrit arm). The primary outcome was time to death or non fatal myocardial infarction. Planned follow-up was 3 years after the last enrolled patient, however it was stopped early by the DSMB (despite not having reached the prespecified stopping boundary). The two arms did achieve a nice separation of hemoglobin, mainly because of higher epo (and iron) use in the 'normal' arm:
Figure 1 from Besarab et al, NEJM 1998
So why was the trial stopped early? Because of the potential harm from the 'normal hematocrit' strategy:
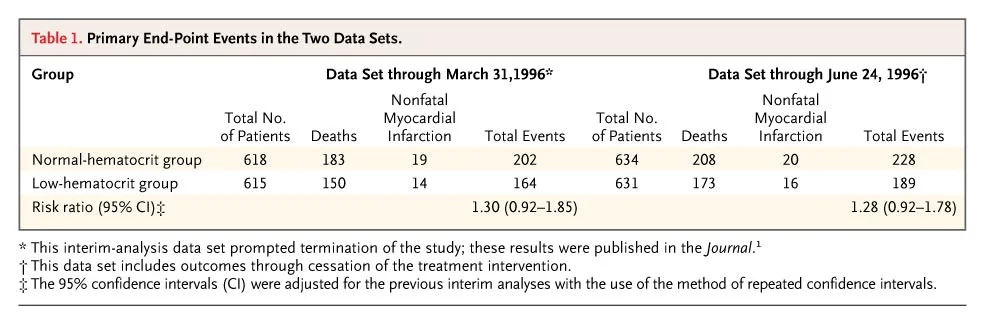
Figure 2 from Besarab et al, NEJM 1998
The separation between the two strategies looks quite significant to the eye, but the stats were not so impressive at this stage: reported risk ratio 1.3 (95% CI 0.9 to 1.9) with 29 months of follow up, as reported in the manuscript. Hence the disappointment and dismay is apparent in the authors write up about the DSMB decision and of the difficulty in interpreting statistical significance. Also, vascular access failure were higher in the normal hematocrit arm. But there is more discussion about the achieved hemoglobin analysis (those who achieved a higher hemoglobin in the normal hematocrit arm had better survival). Also, more iron (which was IV iron dextran in that era) was used in the normal hematocrit arm - and was associated with a higher mortality (RR 2.4, p < 0.001). So perhaps, the higher adverse events were from the higher iron use - and higher achieved hemoglobin was not that bad - and perhaps better. At least, that was the spin that circulated, and in fact emphasized as such in the accompanying NEJm editorial.
After the publication of CHOIR and CREATE almost a decade later, there was some more discussion of the event rates, and the data as reported to FDA. and a letter to NEJM by the authors followed. They presented analysis of the follow up dataset, and re-affirmed that a hematocrit of 42 should not be recommended. See table below from that letter:
From Besarab et al, NEJM 2008, note ++ last footnote about adjustment of 95% CI
How about the QoL results? See how you interpret this:
Screencap from Besarab et al, NEJM 1998, Results section
The hematocrit aim in the control arm was 30; in the normal hematocrit arm was 42. Does the framing of the sentence above make you think that the QoL in the intervention/normal hematocrit arm was significantly better than the control? Most of the readers thought so. And things lay there until 2012.
The Normal Hematocrit Study Revisited: Coyne et al in Kidney International 2012
Daniel Coyne reanalysed the data from the Normal Hematocrit trial, armed with an FOI request to the FDA, and came up with some interesting findings:
The '95% CI' reported for the primary outcome, time death and non-fatal MI, were actually 99.92% CI (perhaps to take into account early stoppage?) The only hint to how these results were reported as 95%CI lies in that 3rd footnote - that these are 'adjusted' for repeated CI to account for interim analyses. These adjusted results, however, resulted in a watering down of the significantly worse outcomes with normal/high hematocrit into the higher, but not significant territory. So the actual results, as analyses by Coyne with the raw data were: RR 1.28 (95% CI 1.06 to 1.56. p 0.0112) for normal hematocrit as compared to controls.
There was a significant increase in the risk of death, alone (RR 1.27, 95% CI 1.04 to 1.54) as well as non-access thrombotic events and hospitalization.
And the so-called quality of life improvement with normal hematocrit? See:
Table 2 from Coyne et al, Kidney International. 2012
So where do the significant improvement in physical function reported in the 1998 NEJM Besarab report come from? As best as we can make out, they are an extrapolation only at best (curiously using the 30 and 42 hematocrit figures to make it seem as if the differences are in the two groups), and specifically an extrapolation from achieved hematocrit data- not randomized hematocrit data. Needless to say, this re-analysis is worth reading carefully and so is the subsequent correspondence in the pages of Kidney International.
Back to the Present: 2016 and a Systematic Review
We return back to the team of Canadian Investigators, from Manitoba, which published this systematic review in the Annals. This was specifically targeted at the QoL outcomes - an earlier systematic review from Suetonia Palmer, et al. in 2010 had reported a higher risk of stroke, hypertension and vascular access thrombosis with higher hemoglobin targets. The measures of QoL examined are Short Form Health Survey (SF-36, meaningful change 5 points) and the KDQ (clinically meaningful change 0.5 points).
The trials included had to be
Treatment of anemia with ESAs in patients with CKD who were re- ceiving or not receiving dialysis.
ESA treatment strategies to achieve 2 hemoglobin targets: a combined placebo or low-hemoglobin target versus a higher hemoglobin target.
Only prospective RCTs
No limits on sample size, duration of follow-up, type of ESA, or use of iron;
Reported in English.
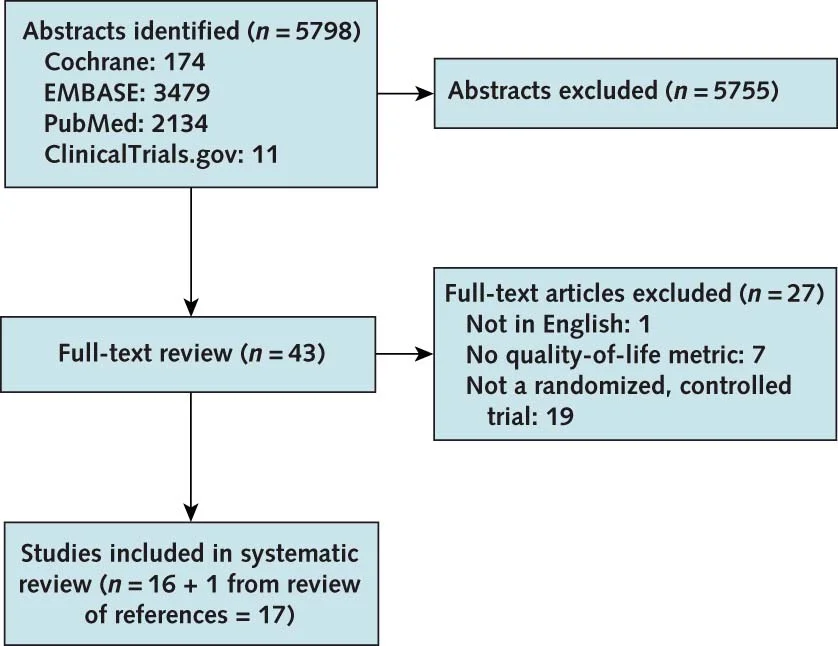
Flow of studies included from Collister et al, Annals 2016
So, the literature search retrieved 17 trials, 10,049 patients - 12 studies of CKD no dialysis, 4 in dialysis patients, and one with a mixed population. Some were with erythropoietin, some with darbepoietin, and the comparators also varied (placebo, controls, lower hemoglobin targets) (see Appendix, tables 3 to 5).
And what did the results show?
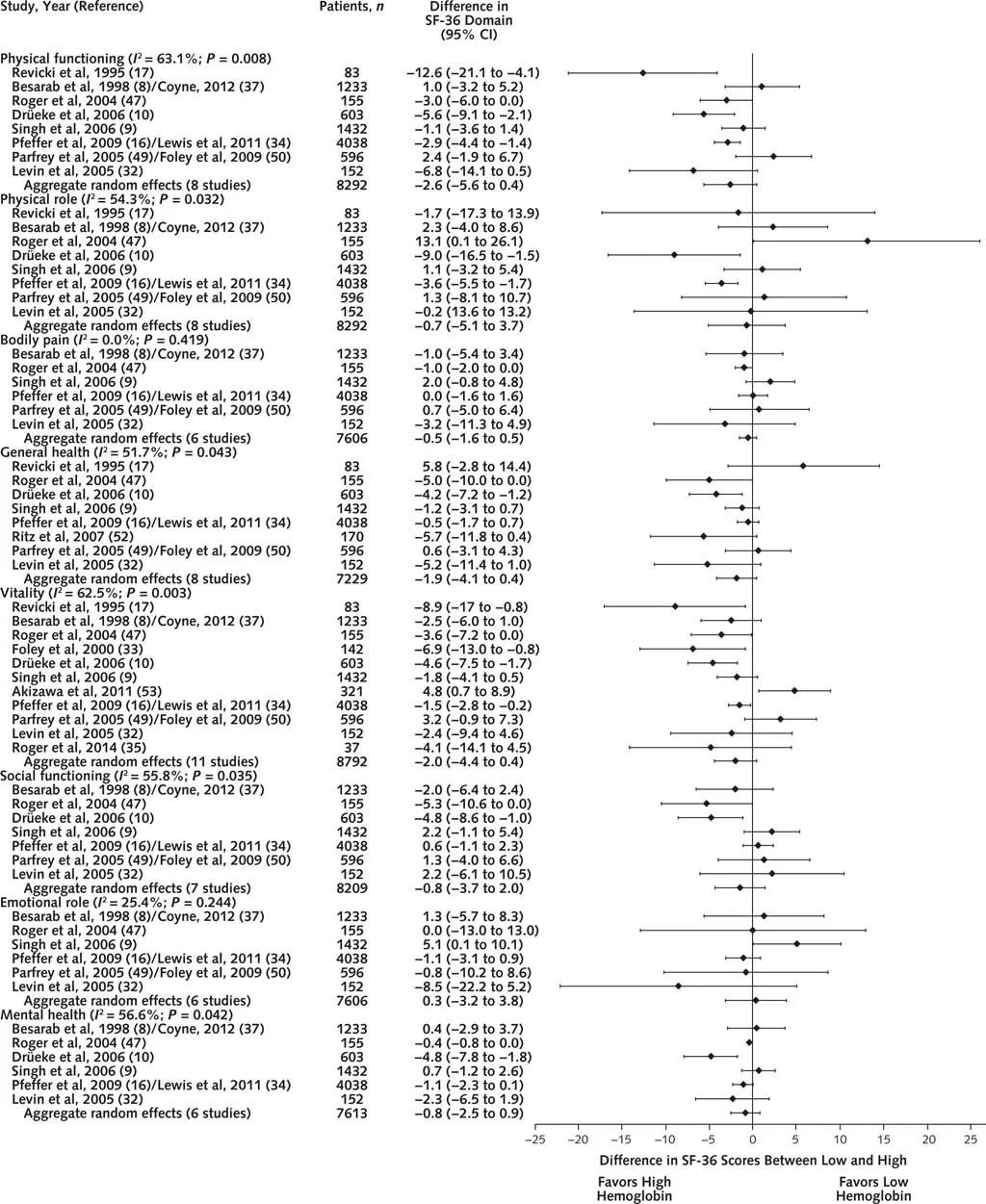
Figure 1 from Collister et al, Annals 2016
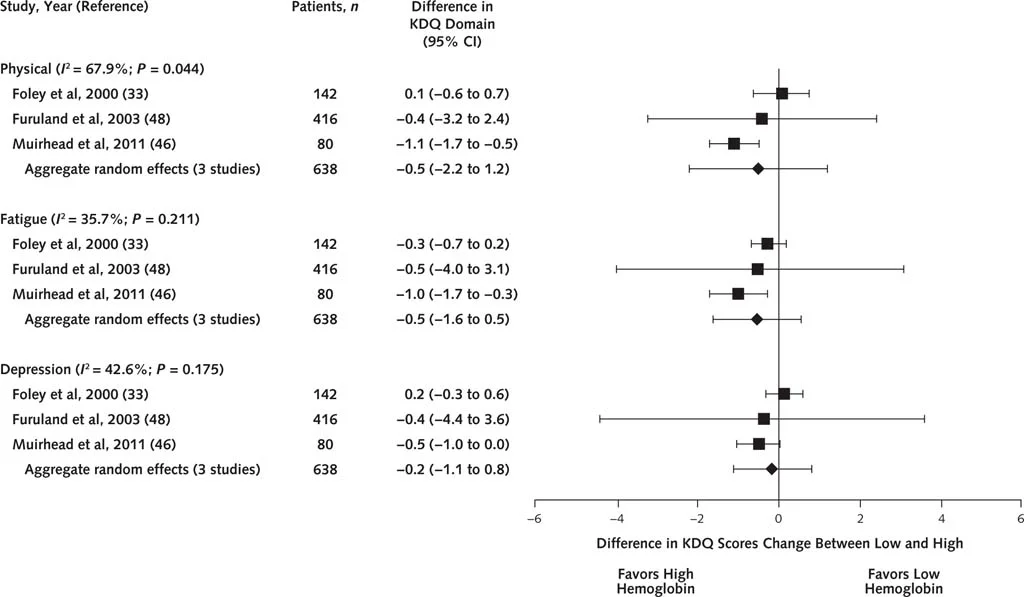
No difference in any of the SF-36 domains. Similar results with the KDQ:
Figure 2 from Collister et al, Annals 2016
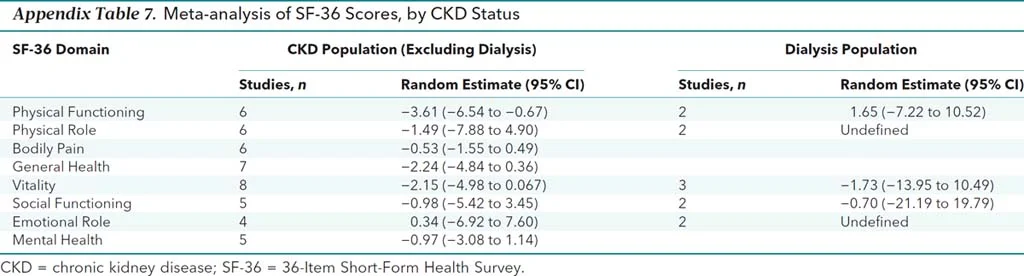
The authors did do a number of subgroup analyses, with no difference in most results. There was a statistically (3.6 points) - but not clinically significant (= 5 points) difference in one domain in one subgroup (see below)
Appendix Table 7 from Collister et al, 2016
So, after all these years, and so many trials, this is pretty robust data suggesting the lack of any effect on quality of life with erythropoietin therapy.
Other suggested readings:
Editorial in Kidney International by Steven Fishbane and Jay Wish
WaPo coverage of the controversy
An article in the Scientist from Daniel Coyne's point of view
A re-analysis of the aforementioned Canadian Erythropoiesis Study Group
The #NephMadness 2016 coverage of the whole hemoglobin target issue
Summary written by Swapnil Hiremath
#NephJC chat Tuesday March 29th 9 pm Eastern
Wednesday March 30th 8 pm BST, 1 pm Pacific