#NephJC Chat
Tuesday, November 28th, 2023, at 9 pm Eastern
Wednesday, November 29th, 2023, at 9 pm Indian Standard Time and 3:30 GMT
N Engl J Med 2023 Nov 3. doi: 10.1056/NEJMoa2308550. Online ahead of print.
Sparsentan versus Irbesartan in Focal Segmental Glomerulosclerosis
Michelle N Rheault, Charles E Alpers, Jonathan Barratt, Stewart Bieler, Pietro Canetta, Dong-Wan Chae, Gaia Coppock, Ulysses Diva, Loreto Gesualdo, Hiddo J L Heerspink, Jula K Inrig, Gianna M Kirsztajn, Donald Kohan, Radko Komers, Laura A Kooienga, Kenneth Lieberman, Alex Mercer, Irene L Noronha, Vlado Perkovic, Jai Radhakrishnan, William Rote, Brad Rovin, Vladimir Tesar, Hernán Trimarchi, James Tumlin, Muh Geot Wong, Howard Trachtman; DUPRO Steering Committee and DUPLEX Investigators
PMID: 37921461
Introduction
Focal segmental glomerulosclerosis (FSGS) is a pathologists description, but clinically includes primary (previously called idiopathic) FSGS, secondary FSGS with known etiologies (e.g. viral infections, drugs, APOL1, other genetic causes), and FSGS of undetermined cause. FSGS is a leading cause of proteinuria and chronic kidney disease (CKD), accounting for 40% of adult nephrotic syndrome cases and 20% of pediatric nephrotic syndrome. The cornerstone of conservative care medications lower BP and proteinuria, namely renin-angiotensin system inhibitors (RASi), though other possible disease-targets are shown below.
On the immunosuppressive side there have been multiple observational and uncontrolled trials investigating prolonged prednisone therapy vs. pulse oral dexamethasone, and a handful of randomized controlled trials for medications such as cyclosporine coupled with low-dose prednisone. In 2011 an RCT (Gipson et al, Kidney Int 2011; n=192) compared the efficacy of cyclosporine to oral dexamethasone and mycophenolate mofetil, and reported no difference in rates of proteinuria remission in those with steroid-resistant FSGS, which is on par with bleak outcomes of other FSGS studies. This was the biggest trial in FSGS, until now…
With so many new medications in the nephrology realm, could there be a treatment that is better directed toward the pathophysiology of FSGS-related kidney damage? In disease models of FSGS, angiotensin II and endothelin-1, individually and together, have been shown to mediate glomerular injury (e.g. Ortmann et al, Hypertension 2004; Morigi et al, Am J Path 2006). Thus, it is plausible that joint therapy with RASi and endothelin system inhibitors would retard CKD progression in FSGS.
Endothelin 1 (ET-1) is a peptide that plays a role in development of kidney disease and stimulation of kidney cell growth, proliferation, and production of extracellular matrix. ET-1 impacts tubular function, which is mediated by ETa and ETb receptors on vascular smooth muscle, which help control renal hemodynamics. Overproduction of ET-1 leads to glomerulosclerosis and interstitial fibrosis, in addition to inflammation. ET-1 receptor inhibition acts mostly on ETa receptors, and is thought to limit the prosclerotic, fibrinogenic and inflammatory actions of ET-1.
From Komers and Plotkin, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2015
Sparsentan is a dual endothelin and angiotensin receptor antagonist (also known as DEARA), already studied in IgA Nephropathy and discussed on NephJC (though now out of date as was prior to release of the eGFR data!). Following on from a phase 2 trial (DUET, Trachtman et al, JASN 2018), the DUPLEX trial was a phase 3 RCT in FSGS studying not just albuminuria reduction, but also eGFR slope.
The Study
Methods
Study design: Phase 3, multicenter, double-blind, randomized international trial (included patients from North and South America, Asia, Europe and Australia)
Study population: All sites included male and female patients between 18-75 years old, weighing >20 kg at screening. Sites within the United States also included patients aged 8 to 18 years.
Inclusion criteria
Biopsy-proven FSGS, or those with documented genetic mutations in a podocyte protein associated with FSGS
Urinary protein-to-creatinine ratio (uPCR) of >1.5 g/g (>170mg/mmol)
eGFR of at least 30 ml/min/1.73m2 at screening
Exclusion criteria
Secondary causes of FSGS (and patients with BMI >40 kg/m2)
Patients with a history of liver disease, alcohol use disorder, chronic viral hepatitis (HBV/HCV)
Patients with history of coronary disease or heart failure
Patients with certain neoplasias in the last 2 years
Patients with any type of transplant, except cornea
Patient with plans to become pregnant or breast-feed
Randomization: Assigned 1:1 via an interactive web response system to receive sparsentan or irbesartan. Patients were stratified by uPCR (≤3.5 g/g or >3.5 g/g for patients ≥18 years of age, or ≤2 g/g or >2 g/g for patients <18 years of age) and eGFR at screening 30-60 or ≥60 ml/min per 1.73 m2).
Intervention: Patients who were already receiving RASi had a 2-week washout period before randomization. During the doubled-blinded treatment period, randomized participants received sparsentan (target dose 800 mg per day) or irbesartan (target dose 300 mg per day) for up to 108 weeks. The final visit occurred at week 112 (4 weeks after the last dose of the assigned trial drug). Standard-of-care treatment, including RAASi, was resumed during weeks 108 to 112.
Figure S1. Overview of Trial Design from supplementary appendix. From Rheault MN, et al, N Engl J Med. 2023
Endpoints
The primary endpoint was the difference in total eGFR slope. The total eGFR slope was the annualized rate change from day 1 to week 108; this was the primary endpoint in the United States but secondary outside the United States, where the eGFR chronic slope from week 6 to week 108 was accepted as the primary endpoint.
Surrogate efficacy endpoint
The partial remission of proteinuria, defined as a uPCR <1.5 g/g (<170mg/mmol) AND >40% reduction from baseline to week 36 (interim analysis).
Additional endpoints
Exploratory endpoints included the changes in eGFR; change in the uPCR; partial remission of proteinuria; complete remission of proteinuria (defined as a uPCR ratio of <0.3g/g at any time during the double-blind period); 40% reduction in eGFR and kidney failure (defined as initiation of kidney-replacement therapy or a sustained eGFR of <15 ml/min/1.73m2). Composite kidney endpoints included confirmed reduction in eGFR of 40% and 50%, kidney failure, or death.
Safety endpoints included body weight, vital signs, physical examinations, peripheral edema, 12-lead electrocardiograms, and clinical laboratory parameters with a focus on known adverse events (AEs) that have been associated with treatment with ARBs or ERAs such as acute kidney injury, hyperkalemia, hypotension, or markers of fluid retention; changes from baseline in lipid profile; changes from baseline in serum albumin and serum potassium (at each visit); and incidence of treatment emergent AEs (TEAEs).
Analysis:
The study needed 300 patients to have 90% power to differentiate between treatments if the eGFR slope was at least 2.5 ml/min/1.73m2 per year. This itself was derived from an assumption (from the phase 2 DUET trial) that partial remission would be seen in 50% participants on sparsentan and 20% on irbesartan respectively.
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and the modified Schwartz formula for patients aged ≥16 and <16 years at screening, respectively. Efficacy endpoints were tested with the use of a hierarchical (gatekeeping) testing procedure to control the overall family-wise type I error rate.
For the assessment of the exploratory and composite kidney endpoints, 40% and 50% reductions in eGFR were confirmed by a value ≥4 weeks after the initial value indicating a reduction. The relative risks (RR) of reaching kidney failure and the composite kidney endpoints and their corresponding 95% confidence intervals were analyzed using Cochran-Mantel-Haenszel tests controlling for randomization stratification factors.
Funding Source:
Travere Therapeutics designed and funded the trial. An independent steering committee oversaw trial conduct and was responsible for scientific integrity. An independent vendor analyzed the data with oversight from the sponsor. The first draft of the manuscript was written by the first and last authors with assistance from medical writers funded by the sponsor.
Results
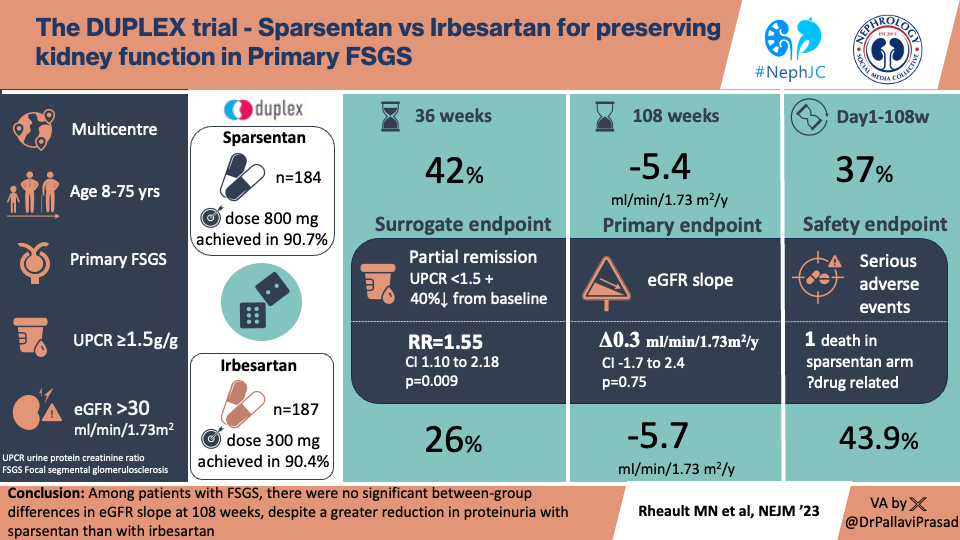
A total of 724 patients were screened for eligibility and 371 underwent randomization; 184 were assigned to receive sparsentan and 187 to receive irbesartan (Fig. S2).
Figure S2. Patient disposition, from Rheault MN, et al, N Engl J Med. 2023
The mean age was 42 (±17) years, and 91% were > 18 years. Women made up 46% of participants, and 74% of the study population were white. The median (IQR) from diagnosis was 2 (±1-6) years, the mean eGFR was 64 (±30) mL/min/1.73m2, the median uPCR was 3 g/g (equivalent to 340mg/mmol), and prior RAASi use was around 80%.
Table 1. Demographic and clinical characteristics of the patients at baseline, from Rheault MN, et al, N Engl J Med. 2023
Efficacy: eGFR slope
Treatment with sparsentan resulted in numerically slower rates of kidney function decline (-4.8 versus -5.7 ml/min/1.73m2 per year in chronic eGFR slope) which was not statistically significantly different compared to the control irbesartan arm. The overall change in kidney function from baseline to the end of the study for patients treated with sparsentan was -10.4 mL/min/1.73m2, compared to -12.1 mL/min/1.73m2 with irbesartan, giving a 1.8 mL/min/1.73m2 higher eGFR at week 108 with sparsentan treatment (Table 2).
Table 2. The eGFR slope and change in eGFR from Rheault MN, et al, N Engl J Med. 2023
Different ways of analysing the eGFR slope data are shown below.
Figure S7 A (total slope) and B (chronic slope) based on different entry criteria Rheault MN, et al, N Engl J Med. 2023
Proteinuria
In the interim analysis at week 36, there was an estimated FSGS partial remission of proteinuria rate of 42% in the sparsentan group versus 26% in the irbesartan group, RR 1.55 (95% CI, 1.10-2.18) (figure 1 panel A). This difference of 18% was lower than the 30% anticipated difference from the pre-trial data.
Figure 1. Panel A. Partial remission of proteinuria at week 36. Rheault MN, et al, N Engl J Med. 2023
Figure 1B shows the change in uPCR over time in both arms.
Figure 1. Panel B. Change in urinary protein-to-creatinine ratio. Rheault MN, et al, N Engl J Med. 2023
Complete remission of proteinuria at any time was more frequent with sparsentan than with irbesartan (18.5% vs. 7.5%; RR 2.47; 95% CI, 1.37 - 4.45) (Fig. 1C).
Figure 1. Panel C. Complete remission of proteinuria at any time from Rheault MN, et al, N Engl J Med. 2023
Prespecified sensitivity analyses that excluded data like initiation or intensification of immunosuppressive therapies were consistent with the primary analyses.
Other exploratory outcomes
Exploratory composite endpoint of eGFR reduction of 40%, kidney failure, or death occurred in 20.1% of sparsentan and in 23% of the irbesartan group (RR 0.87; 95% CI, 0.60 - 1.26). Kidney failure occurred in 6.5% in the sparsentan group and 11.2% in the irbesartan group (RR 0.58; 95% CI, 0.43 - 1.10) (Fig. 2).
Figure 2. Composite kidney end-points from Rheault MN, et al, N Engl J Med, 2023
Blood pressure
In both arms blood pressure was lowered, stabilizing at around week 4. At week 108, BP was 124/78 mmHg in the sparsentan arm and 126/80 mmHg in irbesartan arm (fig. S8). Notably however, hypotension was more common with sparsentan in the adverse event data, shown below.
Figure S8. Mean systolic and diastolic blood pressure by visit from Rheault MN, et al, N Engl J Med. 2023
Safety
Serious treatment-emergent adverse events occurred in 37.0% of the sparsentan group and in 43.9% of the irbesartan group. (Table 3).
Table 3. Adverse events from Rheault MN, et al, N Engl J Med. 2023
Despite the lack of change in BP, more patients had hypotension in the sparsentan arm (17.9% vs 11.2%). Hypotension was a reason to discontinue the treatment for 4 patients in the sparsentan arm (versus none in the irbesartan group).
Although liver function test (ALT/AST) elevations and serum potassium levels were slightly greater in the sparsentan group (0.39 mmol/L versus 0.32 mmol/L), neither directly contributed to discontinuation of treatment medications. Drug-induced liver injury occurred in no patients in the sparsentan group and in 1 patient in the irbesartan group. Acute kidney injury occurred in 4.3% of the sparsentan group (being considered a serious event in 1.6%), and in 7.0% of the irbesartan group (considered a serious event in 4.3%).
Discussion
The authors of the DUPLEX study must be congratulated for performing the largest interventional study to date in patients with FSGS. The findings confirmed that treatment with sparsentan demonstrated a consistent reduction of proteinuria versus an active control arm, though albeit somewhat less than previously seen in the DUET study. Unfortunately the difference in total GFR slope was very small at ~ 0.3 ml/min/1.73m2 per year, which was not statistically significant. The FDA has allowed albuminuria reduction for accelerated approval, while pending GFR slope data. For GFR slope, it is total slope (not chronic slope) that is the FDA approved outcome of choice, based on meta-analysis (Inker et al, Nature Med 2023) that suggest total slope is more strongly associated with CKD progression than chronic slope.
Figure from Brendon Neuen, Twitter.
Regardless of the clinical form of FSGS, conservative treatments are recommended. It was therefore disappointing that 20% of study participants had no baseline use of RAASi, and again shows that there is much work to be done within nephrology with regards to getting the basics right with CKD prescribing.
Sparsentan was also evaluated in the treatment of IgA nephropathy in the PROTECT study, again against the active competitor irbesartan; the proteinuria data was previously discussed by NephJC, but now the final eGFR slope has also been published (Rovin et al, Lancet 2023). The study showed ~40% reduction of proteinuria, and crucially also a slower decline of kidney function in the sparsentan arm; although the total slope difference of -2.9 ml/min/1.73m2 per year just missed statistical significance at p=0.058, the chronic slope difference was statistically different. Also of note PROTECT managed to demonstrate the eGFR benefit despite having relatively slower progression in the irbesartan arm (-9.1 ml/min/1.73m2 over the course of the study, versus 12.1 ml/min/1.73m2 in DUPLEX), and only using half the maximum dose of sparsentan at 400mg once daily. The safety results were very similar to those seen in DUPLEX; numerically slightly more hyperkalaemia, AKI, hypotension, but overall the drug appeared well-tolerated and no excess of LFT abnormalities or edema/diuretic requirement were seen with sparsentan. The rate of eGFR deterioration despite treatment also shows how much more research we need to do to improve outcomes in IgA.
Patients with FSGS are a very heterogeneous group. Lumping all FSGS patients, regardless of etiology, into a treatment group may possibly dilute benefits that could be accrued by some sub-groups. Patients’ FSGS subtypes, degree of fibrosis on kidney biopsy, the need for some to have immunosuppressive therapy during the trial may have influenced the outcomes of the DUPLEX study, but this is uncertain. However, the exact reason why sparsentan convincingly decreased proteinuria more effectively than ARB alone but hasn’t then shown any benefit to eGFR slope like in PROTECT, is unknown. We’ll always be left wondering if there was truly no effect of sparsentan on eGFR in FSGS, or whether the trial had been longer (so there was more time for eGFR slope to improve after the proteinuria had improved) the results would have been different. Unlike sparsentan for IgA nephropathy, it was not fast-track approved by the FDA for approval for FSGS treatment.
In the absence of other effective therapies at present many are turning to flozins, as the C-SMART meta-analysis of patients with glomerular diseases within the larger DAPA-CKD and EMPA-KIDNEY RCTs showed an overall reduction in risk of kidney disease progression by 40%. Unfortunately however, there were small numbers of patients with FSGS and their subgroup appeared to possibly have less favourable results (as much as one can comment upon under-powered subgroup analysis), leading to some calling for their own dedicated FSGS flozin trial.
Figure S5 from Staplin et al, C-SMART analysis, Lancet 2022
Conclusions
This is the largest study ever conducted in FSGS, and sparsentan reduced proteinuria and was well tolerated by patients. Unfortunately there was no beneficial effect on preservation of kidney function based on eGFR slope data over two years.