#NephJC Chat
Tuesday, December 19th, 2023, 9 pm Eastern
Wednesday, December 20th, 2023, 9 pm Indian Standard Time, 3:30 GMT
Eur Heart J. 2023 Nov 7:ehad716. doi: 10.1093/eurheartj/ehad716. Online ahead of print.
Discontinuation vs. continuation of renin-angiotensin system inhibition before non-cardiac surgery: the SPACE trial
Gareth L Ackland, Akshaykumar Patel, Tom E F Abbott, Salma Begum, Priyanthi Dias, David R Crane, Sameer Somanath, Alexander Middleditch, Stuart Cleland, Ana Gutierrez del Arroyo, David Brealey, Rupert M Pearse, the Stopping Perioperative ACE-inhibitors or angiotensin-II receptor blockers (SPACE) trial investigators
PMID: 37935833
Introduction
In the intricate dance of medical decisions, one recurring questions is: Should we stop renin-angiotensin system inhibitors (RASi) perioperatively in non-cardiac surgery patients? In the prior STOP ACEi trial it was demonstrated that withdrawal of RASi in advanced CKD had minimal effect on eGFR, hyperkalemia, and major adverse cardiovascular events (MACE). However, patients undergoing non-cardiac surgery may represent a different population with different risk factors for AKI and hyperkalemia. So, even though we learned to not stop ACEi in advanced CKD, perhaps there is SPACE in the surgical arena for withholding RASi to prevent complications, including AKI and the potential need for dialysis.
Myocardial injury after noncardiac surgery (MINS) is defined as ischemia-induced injury during or within 30 days after surgery. MINS includes postoperative elevation of cardiac enzymes (hsTnT) of at least 20 ng/L or absolute hsTnT change of at least 5 ng/L. Large observational studies revealed that over 1% of patients aged 45 years or older undergoing major non-cardiac surgery die in the hospital or within 30 days of surgery (Smilowitz NR et al, JAMA 2017). The VISION study found that a rise in postoperative hsTnT within the first 3 days after noncardiac surgery is significantly linked to 30-day mortality (Devereaux PJ et al, JAMA 2017). Most MINS cases (94%) occur within 2 days, but only 15.8% have ischemic symptoms, underscoring the need for systematic postoperative troponin monitoring to detect the remaining 84.2%. It is important to note that non-resumption of ACEi within the first 14 postoperative days may unveil a higher postoperative mortality risk: HR 3.44, 95% CI, 3.30–3.60 (Mudumbai SC et al, Journal of Hospital Medicine, 2014).
Perioperative hemodynamic instability in patients using RASi emerges as a potential contributor to MINS, with studies indicating higher risk of hypotension (Hollmann C, et al. Anesth Analg. 2018) with increased inotrope and vasopressor use (PREOP ACEi trial, Shiffermiller JF et al. J Hosp Med. 2018). However, the PREOP ACEi trial also reported more frequent postoperative hypertensive events when discontinuing RASi (RR: 1.95, 95% CI 1.14-3.34). The decision to stop a medication preoperatively hinges on minimizing adverse interactions with anesthesia components. The hemodynamic variation seen during anesthesia is primarily due to the effects of anesthesia on the sympathetic nervous system (Behnia R et.al, Curr Pharm Des 2003). By blunting the effect of the sympathetic system on vascular tone, anesthesia may increase reliance on renin-angiotensin system and endogenous vasopressors to maintain blood pressure.
The first study to assess the effect of preoperative RASi management on the incidence of cardiovascular outcomes, which included MINS distinct from myocardial infarction, was by the VISION investigators (Roshanov PS et al, Anesthesiology 2017). They concluded that withholding RASi before major noncardiac surgery was associated with a lower risk of death and postoperative vascular events. However, because of potential confounders and the observational nature of the VISION study, a large, randomized trial was needed to confirm this finding.
The SPACE trial comes to address the unsettling dilemma: does stopping RASi before elective non-cardiac surgery reduce myocardial injury, MACE, or mortality?
The Study
Methods
The SPACE trial is a randomized, open-label trial following an intention-to-treat principle, with open allocation of the subjects to the study groups. The trial aimed to determine whether continuing RASi through the perioperative period had an impact on the risk of postoperative myocardial injury and morbidity. The only blinded segment of the trial was the primary outcome. (Study protocol)
Study Population
The trial enrolled participants across six centers in the United Kingdom from July 2017 to October 2021. Initially, 1110 subjects were assessed for eligibility. A sample size of at least 260 was targeted to achieve a 90% statistical power aimed at determining a 20% absolute risk reduction.
Inclusion and Exclusion Criteria
Notably, patients with HFrEF were not included. Whether patients with CKD were excluded is uncertain, as there is no mention about the prevalence of CKD or ESRD in the subjects enrolled.
Randomization
Patients were randomly assigned by an internet-based system to the following two arms in a 1:1 ratio:
Stop arm: discontinue RASi before the surgery
Continue arm: continue RASi through the perioperative period
Subjects who did not stop RASi on randomization to that arm, or those who did not restart the medication after the surgery, were automatically withdrawn and were not reincluded.
Intervention
Discontinuation of RASi
The time of cessation was defined by the duration of action of the medication to allow for an adequate “washout” period. If the duration of action was greater than or equal to 24 hours, the drug was stopped 48 hours before the surgery. All other drugs were stopped on the morning of the day before surgery (24 to 32 hours).
Appendix 1. Duration of preoperative cessation required for SPACE trial from Ackland GL et al, European Heart Journal, 2023.
Post-operative resumption of RASi
In the stop arm, all RASi that were held were restarted on postoperative day 2 with any dose modifications. The exceptions to resuming medications on postoperative day 2 were any one of the following:
Systolic blood pressure (SBP) was <90 mmHg
Requirement of vasoactive agents for hemodynamic support
Subjects had sustained acute kidney injury as defined by the Kidney Disease Improving Global Outcomes guidelines (KDIGO AKI Classification).
Follow Up
Patients were followed up for 30 days after surgery for any adverse events.
Endpoints
The primary objective was to determine the occurrence of myocardial injury. This was done by measuring high-sensitivity troponin T (hs-TnT) before induction and at 24 hours and 48 hours after surgery. The primary endpoint was presumed to be achieved under one of these two conditions:
Hs-TnT ≥ 15 ng/L within 48 hours after surgery if the pre-operative values were < 15 ng/L
Post-operative increase in hs-TnT by ≥ 5 ng/L if preoperative values were ≥ 15 ng/L
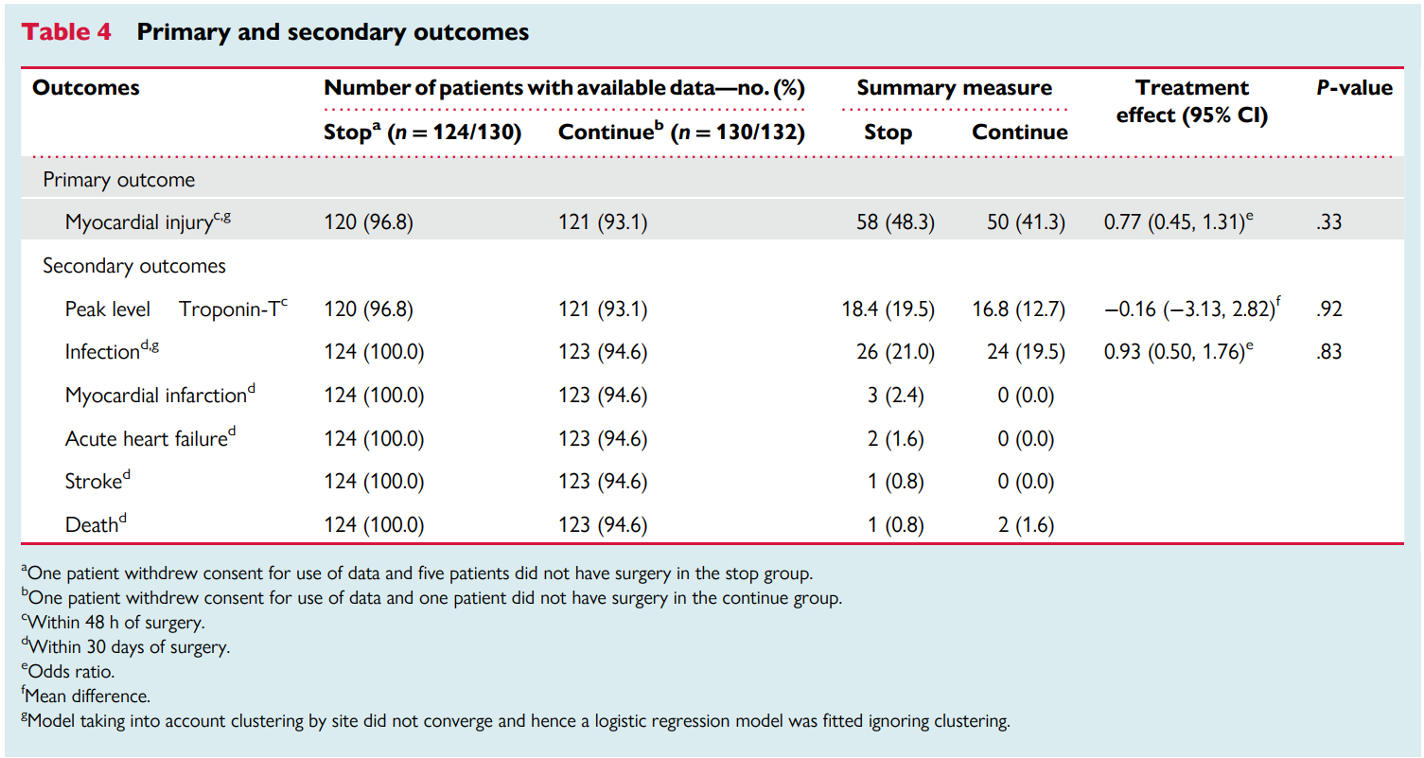
The trial also had secondary endpoints and safety outcomes, as depicted in the table.
Statistical analysis
Assuming a 5% attrition rate, a sample size of 260 patients with 130 in each arm was estimated to achieve a power of 90%. This was aimed to determine an attributable risk reduction of 20% in the incidence of myocardial injury with a type I error of 5%.
The primary endpoint of acute myocardial injury was analyzed using a mixed-effects logistic regression model to estimate the odds ratio (OR) and the 95% confidence interval (CI). The model was adjusted for variables including the type of surgical procedure, the class of RASi (ACEi, ARB, or both), and for covariates- age and sex.
Among the secondary outcomes, peak troponin values were analyzed using a mixed-effects linear regression model adjusted for the above-mentioned covariates while the post-operative infection rate was analyzed using a logistic regression model which was adjusted for minimization variables.
Low event rates precluded analyses of all other secondary outcomes.
Funding
This trial was funded by the British Oxygen Company research chair grant from the Royal College of Anaesthetists, administered by the National Institute for Academic Anaesthesia and the National Institute for Health Research (NIHR). There was no financial support from industry.
Results
A total of 1110 patients were screened from July 2017 to October 2021. 848 were excluded during the initial screening process, the exact details of which are not provided. Among the 262 participants selected for randomization, 130 were assigned to the stop arm and 132 to the continue arm. Only 120 and 121 patients from each of the respective arms were included in the final intention-to-treat analysis. The attrition was due to factors like cancellation of the surgical procedure, self-withdrawal of consent by the participants, and lack of availability of laboratory data to determine primary outcome.
Figure 1. CONSORT diagram from Ackland GL et al, European Heart Journal, 2023.
Baseline demographics of the study population
The mean age was 71 years, and approximately 50% of the population were women. The majority of patients were classified as grade III physical status according to the American Society of Anesthesiology classification- patients with a severe systemic disease. Notably, one third of the patients had active cancer, diabetes, or ischemic heart disease (approximately 20%). There is no mention of CKD or kidney function in Table 1. Also, 60–65% of the subjects were on ACEi and the rest on ARBs. Of note, after randomization to the stop arm, 16 patients (12%) did not restart RASi for concerns not guided by the protocol.
Table 1. Baseline characteristics from Ackland GL et al, European Heart Journal, 2023.
Blood Pressure
At baseline, there was no significant difference in SBP between stop (SBP 140± 21 mmHg, mean arterial pressure 97 ± 12) vs continue arm (SBP 138 ± 20, MAP 95 ± 11). On the day of the surgery, in the stop arm, SBP increased by 16 mmHg (95% CI 10-22 mmHg) and MAP by 10 mmHg (95% CI 6-13 mmHg). No changes were seen in the continuing ACEi/ARBs arm.
The clinical management was similar between both arms. Of note, a higher number of patients in the RASi arm had SBP <90 mmHg (55 vs 40), with similar need for vasopressors, and higher administered volumes of IV fluids in the RASi group.
Table 3. Peri-operative management from Ackland GL et al, European Heart Journal, 2023.
Primary Endpoint
Overall, 241 subjects had proper collection of blood samples. A total of 108 events (~ 45%) of myocardial injury occurred in these patients (50 in the continuation versus 58 in the stop arm), with the difference between groups not being significant [OR of 0.77, 95% CI 0.45 -1.31]. Among patients without a preoperative troponin elevation, 21% (19 events in 90 patients) who continued RASi experienced acute events, while 30% (27 events in 89 patients) occurred in those who discontinued RASi. Of note, 31 subjects in each group had elevated troponins on initial evaluation (hs-TnT > 15 ng/L).
After excluding patients who had regional anesthesia with sedation, the difference in myocardial injury was still not statistically significant (OR 0.68, 95% CI 0.40–1.16).
A sensitivity analysis including patients with missing primary outcome data showed robustness, indicating that even if the data were not missing, the conclusion would remain unchanged.
Table 7. Sensitivity analysis for missing data from Ackland GL et al, European Heart Journal, 2023.
Secondary outcomes
There was no difference in the peak hs-TnT levels in the first 48 hours after surgery. This was confirmed by the Wilcoxon-Mann-Whitney (z score: 0.117). Post-operative infections were similar in the stop and continue arms (26 and 24 patients, respectively; OR 0.93, 95% CI 0.50–1.76). Other secondary outcomes, like myocardial infarction, stroke, and mortality, occurred in fewer than 10 patients.
Table 4. Primary and secondary outcomes, from Ackland GL et al, European Heart Journal, 2023.
Adverse events
A total of 43 adverse events occurred in each of the groups. Hypotension requiring vasoactive support was similar in both the discontinuation (12 events, 9.3%) and continuation arms (11 events, 8.4%). Between randomization and 48 hours post-surgery, patients who discontinued RASi experienced more hypertension (16 vs 7), with an OR of 0.40 (95% CI 0.16-1.00). The number needed to harm was 14. The number of AKI events was similar between the two arms (14 and 12, respectively, in stop and continue arms).
The combination of hyper- and hypotension was more common when RASi was discontinued (21.7% vs 13.0%). The authors mention this was associated with longer hospital stays (though from the supplement, it seems this mislabeled, and if interpreted according to the text, it was 6 versus 8 days, and not significantly different). There was also no significant difference in intraoperative hypotension, whether or not RASi were continued: SBP < 90 mmHg in 55 patients in the continuation vs 40 patients in the stop arm (OR 1.63; 95% CI 0.97 to 2.73). Acute kidney injury in patients with normal preoperative troponin values occurred in 24 out of 183 patients (13%), compared to 16 out of 63 patients with elevated troponin values (25%).
Table 5. Prespecified adverse events from Ackland GL et al, European Heart Journal, 2023.
Discussion
The main finding of the SPACE trial was that the incidence of myocardial injury was similar between patients who stopped and those who continued RASi during the perioperative period. However, patients who stopped RASi did experience more hypertensive events postoperatively. By contrast, there was no difference between groups in the need for vasoactive therapy to treat hypotension within 48 hours of surgery. What may seem physiologically plausible does not always pan out when tested in the arena of a properly conducted randomized trial.
A systematic review that included RCTs and observational studies (Hollman et al, Anesth and Analg 2018) had reported that the continuation of ACEi/ARBs on the morning of noncardiac surgery was associated with increased intraoperative hypotension episodes. A major factor in the interpretation of the previous study was that when RASi were stopped and restarted, it was not clearly defined. If RASi were stopped <24 hours before surgery, it seems very unlikely that their withdrawal would have a meaningful impact on the patient’s blood pressure on the day of surgery. SPACE is the first study to adopt a pharmacokinetically based rationale for stopping RASi before surgery.
This study focused on older patients (>60 years) who were at elevated risk of myocardial injury. Pre-load myocyte injury, produced by the transient increase in blood pressure as a result of stopping of RASi, potentially produces mechanical stretch-induced myocyte injury resulting in raised plasma troponin levels (BR Well et.al, JACC 2018). The post hoc analyses of this study showed that blood pressure lability is more common after discontinuation of RASi, as evidenced by the combination of acute hypertension and intraoperative hypotension.
A similar study by PREOP-ACEI (Shiffermiller et.al, J Hosp Med. 2018) where they had an omission and continuation group of ACEi therapy prior to non-cardiac/non-vascular surgery, found more hypotensive episodes in the continuation group (RR 0.81, 95% CI 0.67–0.97) and more hypertensive events in the discontinuation group (RR 1.95, 95% CI 1.14–3.34). In SPACE study, a higher number of patients in the RASi arm had SBP <90 mmHg (55 vs 40), with a similar need for vasopressors, and higher volumes of IV fluids.
This trial had several limitations, including a lack of minority racial representation. Additionally, the open-label design of the trial may have influenced clinical care. Most importantly, the findings may not be generalizable to patients receiving RASi for indications other than hypertension. It is quite surprising to note that eGFR and chronic kidney disease were not mentioned, as they are a population with extensive RASi usage. Hopefully, the ongoing trials STOPorNOT and the POISE-3 - which included patients with eGFR >15 ml/min, respectively >30 ml/min, will bring more information in the following years.
Our readers might argue that SPACE it’s only a phase 2 trial, but cannot help mentioning that it was underpowered- only 241 participants were considered (vs 260 needed for 90% power). As shown by sensitivity analyses, this might not change MINS outcomes, but for sure it made our hearts stop for a second when we saw the odds ratio and confidence interval for hypertensive adverse events in the stop arm (OR 0.40, 95% CI 0.16-1.00).
What do guidelines suggest? The 2014 guidelines set by the American College of Cardiology and American Heart Association (ACC/AHA) suggest the uninterrupted perioperative continuation of RAS inhibitors and to restart postoperatively as early as possible if it was held before surgery (AHA,2014). The 2022 European guideline, just prepares the ground for a good pro-con debate, but it doesn’t give a clear recommendation if to stop or not RASi before non-cardiac surgery. (Halvorsen S, et al. Eur Heart J. 2022) A 2022 statement consensus recommends withholding the RASi only on the morning of the surgery, a recommendation which doesn’t take into account the individual drug’s pharmacokinetics (Sahai SK, et al.Mayo Clin Proc. 2022) Given the new evidence published since these guidelines need to be revised
Conclusion
Discontinuation of RASi in patients undergoing non-cardiac surgery did not lead to a clinically relevant reduction in myocardial injury and/or other complications but did increase the risk of clinically significant hypertensive events. There is still SPACE for ACEi use in the surgical patient.
Summary by
Sabarinath S MD DM PDF FASN
Consultant Nephrologist and Renal Transplant physician
RMC Superspeciality Hospital, Tiruppur, India
and
PGY3 Internal Medicine Resident,
Nazareth Hospital, Philadelphia, USA
NSMC interns, class of 2023
Reviewed by:
Cristina Popa, Brian Rifkin, Jade Teakell, Swapnil Hiremath


















In a NephJC first, first author, Laurie Tomlinson wrote up some comments on the implications of her study.