#NephJC Chat
Tuesday June 11, at 9 pm Eastern
Wednesday June 12 9 pm Indian Summer Time
Wednesday June 12, 9 pm BST, 1 pm Pacific
Lancet. 2019 May 11, doi: 10.1016/S0140-6736(19)30772-X
Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial.
Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators.
PMID: 30995972
Introduction
Approximately 422 million people are living with diabetes worldwide, with the global burden expected to double between 2000 and 2030. Diabetic kidney disease occurs in approximately 40% of patients with diabetes, and is the most common cause of end stage kidney disease (ESKD), accounting for nearly 44% of incident cases in the US.
Blocking the renin–angiotensin–aldosterone system (RAAS) represent the principal therapeutic intervention based on landmark trials demonstrating that angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) effectively reduce proteinuria and slow progression of diabetic nephropathy. However, even with RAAS blockers, people with diabetes and chronic kidney disease (CKD) remain at high risk of developing ESKD and cardiovascular (CV) complications, particularly when high concentrations of albuminuria persist. [6]
Background evidence for Endothelin receptor antagonists
The mainstays diabetic nephropathy treatment is blood pressure (BP) control and RAAS blockade with an ACEI or ARB. Prior to CREDENCE, no additional therapy that lowers albuminuria had been shown to improve long-term renal outcomes. Excessive activation of the renal endothelin system, particularly renal endothelin-1, is a potent mediator of kidney injury in diabetes; thus, endothelin antagonism may be nephroprotective in diabetes. Prior clinical trials, in which all patients received RAAS blockers, have shown that endothelin receptor antagonists (ERAs) further reduce residual proteinuria.
Unfortunately, ERAs have a high incidence of peripheral edema and other adverse events related to fluid retention. A previous large RCT using avosentan, a non-selective ERA, in patients with diabetes and CKD was stopped prematurely because of an increased incidence of heart failure. Compared to avosentan, atrasentan is a more selective ERA, which in short-term studies reduced albuminuria with minimal sodium retention in patients with type 2 diabetes and CKD (NephJC coverage here, our second NephJC ever). These preliminary findings lead to this phase 3 clinical trial to establish whether atrasentan can delay progression to ESKD.
Key points for Endothelin receptor antagonist treatment:
Preclinical studies show that selective blockade of the Endothelin A (ETA) receptor downregulates the fibrotic, inflammatory, and proliferative actions of endothelin-1
Phase 2 clinical trials provide evidence that ETA receptor antagonists reduce residual proteinuria in patients with overt diabetic nephropathy who are already on maximal RAAS blockade
The clinical use of ETA receptor antagonists has been associated with excess risk of peripheral edema and worsening of heart failure
The Study
Methods
Study Design
Randomized, double-blind, placebo-controlled, phase 3 trial at 689 sites in 41 countries
To enhance the likelihood of detecting a treatment benefit while minimizing the risk of heart failure, the trial used an enrichment design.
Inclusion Criteria:
Adults aged 18–85 years with type 2 diabetes
eGFR of 25–75 mL/min/1·73 m²
Urine albumin-to-creatinine ratio (UACR) of 300–5000 mg/g
Serum albumin ≥ 25 g/L
Brain natriuretic peptide (BNP) concentration ≤ 200 pg/mL
Serum potassium of 3.5-6.0 mmol/L
Systolic BP of 110–180 mmHg
Treatment with a stable, recommended (or maximally tolerated) dose of an ACEI or ARB was required for at least 4 weeks before entry into the enrichment period
Exclusion Criteria:
diagnosis of or previous hospital admission for heart failure, a history of severe peripheral or facial oedema,
diagnosis of type 1 diabetes,
history of pulmonary hypertension, pulmonary fibrosis, or any lung diseases requiring oxygen therapy, and
known non-diabetic kidney disease.
Enrichment-responder design
Participants who met the inclusion criteria for initial study entry and none of the exclusion criteria were eligible to proceed to the Run-In Period to optimize RAS inhibitor and diuretic doses. Following the Run-In Period, 4711 eligible participants entered the 6-week ‘Enrichment Period’ in which all received 0.75 mg/day oral atrasentan to determine their UACR response and to assess tolerability of atrasentan. In this enrichment period, patients who developed evidence of fluid retention were excluded, in an attempt to minimise the risk of heart failure. After 6 weeks, 2648 responders (~ 55%) to treatment, with a UACR decrease of at least 30% and with no substantial fluid retention were included in the double-blind treatment period. These responders comprised the primary analysis population used to establish the efficacy and safety of atrasentan.
To date, this is the first clinical trial in patients with type 2 diabetes to use an enrichment-responder design. It does represent a carefully selected subpopulation of diabetic patients at low risk of adverse effects and with higher chance of benefit with the drug.
Outcome measures
Primary Outcomes:
Efficacy of atrasentan in delaying the progression of CKD, defined as the time from randomization to the first occurrence of any of the following components of a composite endpoint in the Intent-to-Treat (ITT) Responder Set:
Doubling of serum creatinine
Onset of end stage renal disease (eGFR < 15 ml/min/1.73 m^2 confirmed by a 90-day eGFR measurement)
Receiving chronic dialysis
Renal transplantation
Death from kidney failure.
Secondary Outcomes:
Time to at least 50% eGFR reduction in the ITT Responder Set
Time to Cardio-renal Composite Endpoint in the ITT Responder Set consisted of doubling of serum creatinine, ESRD, CV death (including CV death and presumed CV death), nonfatal myocardial infarction (MI; heart attack) and nonfatal stroke.
Time to First Occurrence of a Component of Composite Renal Endpoint for All Randomized Participants (responders and non-responders combined) renal endpoint in all randomly assigned patients
Time to the CV Composite Endpoint in the ITT Responder Set defined as CV death, non-fatal MI, or non-fatal stroke.
Time to hospital admission for heart failure in responders and time to the primary renal outcome in non-responders were additional prespecified outcomes
Statistics simplified
The trial was event-driven – meaning that the study was driven by the occurrence of the primary outcome rather than being of fixed observation time. The investigators estimated that 425 events were needed to detect a 27% risk reduction (hazard ratio [HR] 0.73) with 90% power using a two-sided α level of 0.05, assuming an annual rate for the primary renal outcome of 6% in the placebo group. However, after all patients were randomly assigned, it became apparent that the rate of the primary composite outcome was much lower than expected and that the time needed to accrue 425 events would be much longer than expected. The sponsor decided to stop the trial prematurely, before the planned interim analysis was completed.
At completion of the trial, 184 primary renal events had occurred providing more than 90% power to detect an HR of 0.62 and 80% power to detect an HR of 0.66 with a two-sided α level of 0.05.
Funding
The funder, AbbVie, participated in study design, data collection, data analysis, data interpretation, and reviewing and approving the manuscript, but was not involved in the writing of the report. Three of the authors are AbbVie employees, including one who did the analysis (TY).
The study is registered with ClinicalTrials.gov, number NCT01858532.
Results
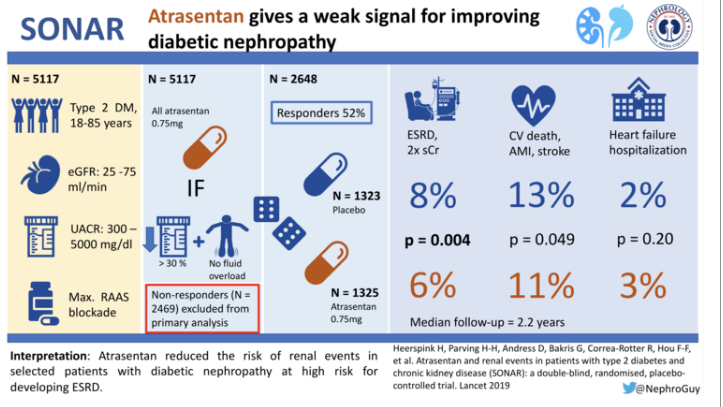
Between May 17, 2013, and July 13, 2017, 11,087 patients were screened; 5117 entered the enrichment period, and 4711 completed the enrichment period. Of these, 2648 patients were responders and were randomly assigned to the atrasentan group (n=1325) or placebo group (n=1323).
Figure 1 from Heerspink et al, Lancet 2019
The baseline characteristics are shown in table 1 (below). Remarkably, 75% of them were men, with about a third being Asian but only 5% being black. Mean duration of diabetes was about 16 years, and a third of them had retinopathy. The mean blood pressure was not bad (~ 136/75) and ARBs:ACEi were used in a 2:1 ratio overall. Mean GFR was ~ 45 and the median ACR was 800 mg/g. Also note the low BNP levels. Other features can be seen below.
Table 1 from Heerspink et al, Lancet 2019
During follow-up, 260 (19.6%) of 1325 patients in the atrasentan group and 251 (19.0%) of 1323 in the placebo group discontinued treatment prematurely.
Before jumping into the outcomes, lets see the effects on the intermediaries
Figure 2: from Heerspink et al, Lancet 2019
So, atrasentan reduces blood pressure somewhat, and albuminuria even more. Interestingly, BNP went up in both groups and so did body weight. But does a change in albuminuria matter?
Primary Outcomes
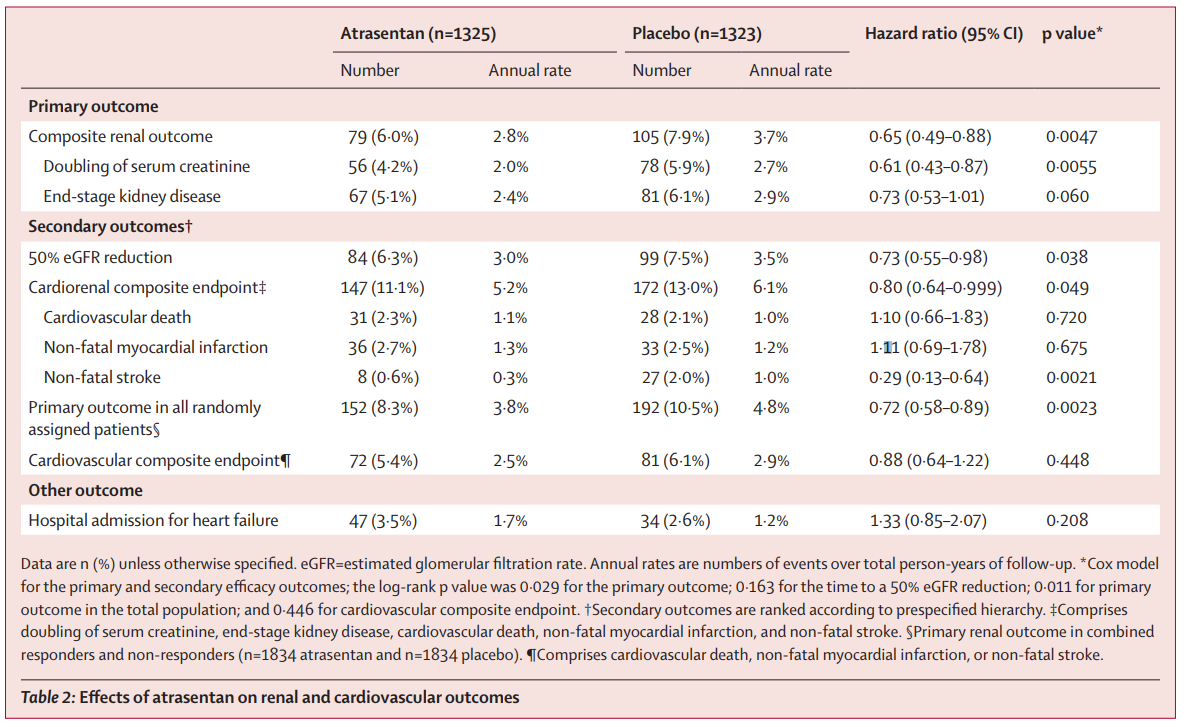
Over a median-follow up of 2.2 years (IQR 1·4–2·9), 79 (6.0%) of 1325 patients in the atrasentan group and 105 (7.9%) of 1323 in the placebo group had a primary composite renal endpoint event (HR 0.65 [95% CI 0·49–0·88]; p=0·0047) These results translated into annual rates of 2.8% and 3.7%, respectively, and a significant HR of 0.65 favoring atrasentan.
All endpoints are shown in table 2
Table 2 from Heerspink et al, Lancet 2019
The figure below shows the renal specific endpoints: composite, followed by doubling and ESKD:
Figure 3. Effects of atrasentan on the primary composite renal outcome and its components in responders
A: Composite primary renal outcome
B: doubling of serum creatinine
C: end-stage kidney disease
From Heerspink et al, Lancet 2019
Secondary Outcomes
Rates of hospital admission for heart failure (3.5 vs 2.6%) and death (4.4 vs 3.9%) were also numerically higher in the atrasentan group, but the difference did not reach statistical significance. Hospital admission for heart failure occurred in 47 (3.5%) of 1325 patients in the atrasentan group and 34 (2.6%) of 1323 patients in the placebo group (HR 1.33 [95% CI 0.85–2.07]; p=0.208). 58 (4.4%) patients in the atrasentan group and 52 (3.9%) in the placebo group died (HR 1.09 [95% CI 0.75–1.59]; p=0.65).
Participants in the atrasentan group were significantly less likely to experience a decline in eGFR of at least 50% (HR=0.73) and had a significantly lower risk for the cardiorenal composite outcome of doubling of serum creatinine, end-stage kidney disease, cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke (HR=0.80) compared with those given placebo.
When the atrasentan non-responders in 6-week Enrichment Period combined with responders (n=3668), the primary renal outcome occurred in 152 (8.3%) of 1834 patients in the atrasentan group and 192 (10.5%) of 1834 patients in the placebo group (HR 0.72 [95% CI 0.58–0.89]; p=0.0023; figure 4, table 2).
Safety
Serious adverse events occurred significantly more frequently among people treated with atrasentan versus placebo (36.3 vs 32.6%), as did the treatment-emergent adverse events of fluid retention (36.6 vs 32.3%) and anemia (18.5 vs 10.3%), which the investigators say “have been previously attributed to endothelin receptor antagonists.” The higher adverse events with atrasentan occurred despite selection of patients at low risk of these outcomes (eg low BNP) and the ‘enrichment’ period which screened out patients who had immediate adverse events.
Table 3 from Heerspink et al, Lancet 2019
Discussion
Taken together, the authors report that these results support ETA receptor antagonism as a therapeutic for patients with type 2 and CKD. Atrasentan reduced the risk of renal events in patients who were selected to optimize efficacy and safety.
Before we start prescribing atrasentan lets ponder over a few aspects of the trial:
Enrichment and Run-in
The investigators highlight the run-in period and enrichment as a strength. A glass half full is also a glass half empty. So in some respects it is a strength: endothelin antagonists are not benign molecules to be prescribed without some thought. They cause sodium retention. This does not bias the study itself, but it makes generalizability of the trial extremely weak. Of the 11,000 patients screened, only half made it to enrollment, and a quarter were actually included in the intention to treat analysis. From figure 1, 258 of the patients in the enrichment withdrew because of adverse events. That was an immediate adverse event, comapred to the puny effect size (absolute difference in composite renal events is about 26, after 3.5 years). There is extensive literature that the use of an active therapy run-in period exaggerates the true effect of an intervention. In the words of a famous gadfly, ‘In short, run-in periods exclude intolerant and nonadherent patients, foster spuriously large treatment effects, and (most troubling) create inclusion criteria that are irreproducible — i.e., that apply to no population we can clearly describe, as reasons for dropout are multifaceted and unique.’
Early Stoppage
The trial was stopped after only 180 of the 425 planned events occurred - mostly related to the low event rate. Could it be that that excluding patients at high risk of atrasentan-related side effects also excluded the patients at high risk of kidney failure? Early stoppage for benefit almost always biases the results unless done very carefully with prespecified iron-clad protocols - though one should grant that this trial was not stopped early for benefit, but purely on the futility given low event rates.
Further, vital status of some patients lost to follow-up remained unknown despite the trial close-out procedures being performed according to the protocol. Although these patients were similarly distributed across the placebo and atrasentan groups, this potential bias has to be taken into account in the trial interpretation. Additionally, during the trial, 19% of patients discontinued their assigned treatment.
Background therapy
In this trial, background therapy was maximally tolerated ACEi or ARB, which is reasonable when the trial was planned. However, there is a new kid on the block, and there is little doubt that patients like these should be on an SGLT2i. What is the effect of atrasentan when added to an SGLT2i and RAS blockade?
Role of Funder
The sponsor had a major role in all aspects of the trial. This is not a limitation per se but rather something to be kept in mind when reading the trial conduct, including the limitations discussed above and the early stoppage etc.
Conclusion
This was a very important trial and full accolades to the authors for planning and conducting this very study - and to the sponsor for following through from RADAR to SONAR. Despite its promise, however, given these limitations, it does not seem like there is truly a clear benefit with atrasentan. There might be a small benefit in a very small and select populations, but the clear increase in adverse events (even discounting the enrichment period) is concerning enough that should give us pause before using this drug. Back to the drawing board on this one.
Summary by Katie Wang, Nephrology Fellow at Stanford and
Yasar Caliskan, Nephrology Fellow at Saint Louis University School of Medicine
NSMC interns, Class of 2019