#NephJC Chat
Tuesday, Oct 3rd, 2023, at 9 pm Eastern (AEST = Oct 3rd, 11 am)
Wednesday, Oct 4th, 2023, at 9 pm Indian Standard Time and 3:30 pm GMT (AEST = Oct 4th, 1:30 am)
Nature Medicine 2023 August 28; 24(2):385-392.
doi: 10.1038/s41591-023-02532-z.
Natriuresis-guided diuretic therapy in acute heart failure: a pragmatic randomized trial
Jozine M Ter Maaten , Iris E Beldhuis, Peter van der Meer Jan A Krikken, Jenifer E Coster, Wybe Nieuwland, Dirk J van Veldhuisen, Adriaan A Voors, Kevin Damman
PMID: 37640861
Introduction
Acute heart failure (AHF) is a growing healthcare burden and one of the leading causes of hospitalizations and readmissions. Approximately 64 million adults worldwide are diagnosed with heart failure, and the projections are that the prevalence of heart failure will increase by approximately 45% from 2012 to 2030 (Mozaffarian D et al., Circulation. 2014). AHF is one of the leading primary diagnoses for hospitalization, with an estimated 1 million US patients discharged in 2010. In addition, around 30% of the patients are rehospitalized within 60 to 90 days of discharge (Gheorghiade M et al., J Am Coll Cardiol 2013). Given the widespread burden on healthcare systems, optimized management of AHF is needed to curtail hospitalizations. Therefore, treatment of AHF is directed toward achieving adequate decongestion using various combinations of diuretics. Loop diuretics are most commonly used as they eliminate extracellular sodium and water from the body; extracellular sodium excess can drive volume overload and congestion (Mullens W, Eur J Heart Fail 2019). Despite high-dose loop diuretics, many patients are discharged from the hospital with residual volume overload. For example, in the Diuretic Optimization Strategies Evaluation (DOSE) trial, only 15% of the patients were free from clinical congestion after 72 hours of treatment. Moreover, in the Acute Decompensated Heart Failure National Registry (ADHERE), approximately 20% of the patients were discharged with increased body weight. As nephrologists, we are often concerned about overly aggressive diuresis leading to intravascular volume depletion and worsening renal function, especially if diuresis occurs at a rate greater than the extravascular fluid can refill the intravascular space. Despite this relationship, hemoconcentration and even a rising creatinine have been associated with substantial improvement in patient survival. These observations raise the question of whether aggressive decongestion can positively affect patient mortality and other outcomes (Testanti JM et al., Circ 2010). So, if adequate decongestion to prevent rehospitalization and reduce excess mortality is the goal, what are the best strategies to guide physicians hoping to maximize a patient’s diuretic response?
An even more basic is the question: what is the best marker for adequate diuresis? Is it a change in body weight? Is it cumulative volume loss over time as measured by urine output? Is it an improvement in symptoms of dyspnea? Dyspnea is the most common complaint prompting patients with AHF to seek emergency care. As a surrogate marker of clinical improvement, however, change in dyspnea severity may be an inadequate measure of response to therapy, given that patient-reported symptoms are highly subjective (Kociol RD et al., Circ Heart Fail, 2013). Studies have shown that weight loss often isn’t an accurate response measure, and post-discharge changes in body weight only predict rehospitalization and not mortality, highlighting the limitations of examining body weight alone (Valente MA et al., Eur Heart J 2014). In fact, weight loss, fluid loss, and NT-proBNP reduction at 72 hours are poorly correlated with symptomatic relief. However, favorable improvements in each of the three markers were associated with improved clinical outcomes at 60 days (McCallum W et al., JACC 2020). Finally, although more objective measures in AHF, including lung point-of-care ultrasound (POCUS), have an established role in diagnosing AHF, their use in AHF management is undefined. Several studies have investigated the application of serial lung ultrasounds to detect dynamic changes in pulmonary edema (Martindale J, AJEM 2016), but have yet to be tested for clinical outcomes.
On the other hand, assessing natriuresis could be a cheap, easy, and objective measure for guiding adequate diuretic therapy. Sodium is the primary osmolyte in the extracellular fluid compartment, and the kidneys regulate total body sodium to adjust total body fluid content. Previous studies have shown that in patients with AHF and symptoms of volume overload who received intravenous diuretics on admission, serial assessments of spot urine sodium (UNa) during the first 48 hours of inpatient admission may provide clinical and prognostic guidance (Biegus J et al., Eur J Heart Fail 2019). A poor natriuretic response, considered to be a UNa output of < 50 mmol/L in a spot urine sample, could direct the need for additional diuretic dosing (Testani JM et al., Circ Heart Fail 2016). In one study, the authors developed a natriuretic response prediction equation (NRPE), which predicted sodium output using a spot urine sample collected 2 hours after loop diuretic administration. The study results highlighted that a natriuretic response can be rapidly and accurately predicted by the NRPE, which could also be used to guide further diuretic therapy (Rao VS et al., J Am Coll Cardiol 2021). Most importantly, it was noted that sodium excretion was found to be strongly associated with 6-month mortality, whereas traditional fluid-based metrics were not. Thus, it was concluded that poor sodium excretion following diuretic administration predicted a worse overall prognosis (Hodson DZ et al., JACC Heart Fail 2019), including a greater likelihood of worsening renal function and future adverse long-term outcomes (Singh D et al., J Card Fail 2014).
Still, limited data suggests that using natriuresis to guide therapy in AHF may be beneficial. The current trial, PUSH-AHF, aimed to provide randomized, high-quality evidence for using UNa as a metric for guiding treatment in patients with AHF.
The Study
The PUSH-AHF trial was a prospective, pragmatic, open-label, randomized controlled trial (February 2021 to November 2022) conducted at the University Medical Center Groningen, the Netherlands.
PRECIS-2 wheel diagram for the PUSH-AHF study.PRECIS-2, Pragmatic Explanatory Continuum Indicator Summary 2
Figure 4, from Ter Maaten JM et al., Eur J Heart Fail 2022.
The study randomized participants to either the intervention group or the standard of care (SOC) group. Randomization was done using a random number generator in the Electronic Health Record (EHR). For patients in the intervention group, a study-specific order set of timed spot urinary sodium collection was generated, coinciding with the start of IV loop diuretic administration. In this study, both the researchers and participants were aware of the treatment administered. However, they were blinded to all urinary sodium measurements.
Study population
The study screened 376 participants, comprising both representation and all-comer patients diagnosed with AHF. Following applying specific inclusion and exclusion criteria, 310 individuals met the eligibility criteria and were included in the study. The criteria used to select the study population are listed in the figure below.
Illustration of the inclusion and exclusion criteria.
Intervention
The authors used the Heart Failure Association of the European Society of Cardiology recommendations on natriuresis-guided diuretic therapy. The initial dose of IV bumetanide was based on eGFR and outpatient usage, with a maximum of 5 mg.
Starting dose of loop diuretics (in all patients); Supplementary table S1 from Ter Maaten JM et al., Nat Med. 2023.
The initial bumetanide dose was then continued twice a day (every 12 hours dosing schedule). For the SOC group, diuretic dosage adjustment was left to the discretion of the physician. However, for the natriuresis-guided treatment group, spot urine samples were taken at 2, 6, 12, 18, 24, and 36 hours. If the levels of urinary sodium or diuretic output (except at the initial 2-hour measurement) were considered inadequate (UNa <70 mmol/L and/or Urine volume <150 ml/hr), then decongestive therapy was modified based on predefined orders. The treatment plan involved administering an additional dose of loop diuretic, double the previous dose with a maximum of 5 mg of bumetanide. If there was inadequate natriuresis or diuresis despite the additional diuretic dose, then combination therapy was initiated. This included adding 25 mg of hydrochlorothiazide, followed by acetazolamide (500 mg once daily), an SGLT2 inhibitor, and finally ultrafiltration as a last-resort. After 48 hours, diuretic adjustments were at the physician’s discretion. Additionally, patients were contacted after 180 days for updates on vital status and regarding any perceived significant adverse events.
Treatment protocol in the natriuresis guided arm during the first 24 hours (0-24 hours after randomization) ; Supplementary figure S1 from Ter Maaten JM et al., Nat Med. 2023.
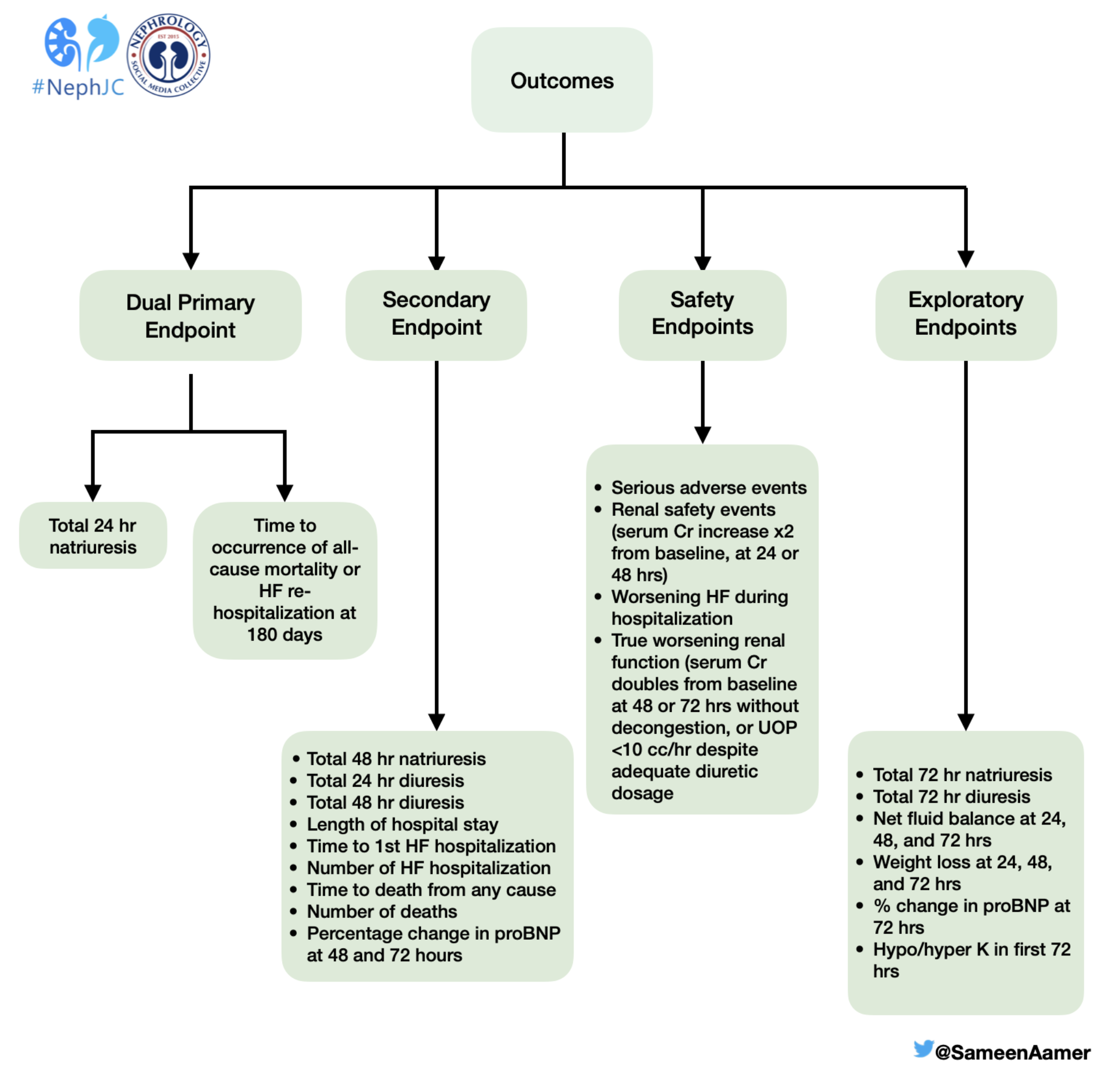
Outcomes
The primary outcome of the study was a combination of two co-primary endpoints: the total 24-hour natriuresis and the first occurrence of either all-cause mortality or rehospitalization due to heart failure within 6 months. The study also looked at secondary, safety and exploratory endpoints. Check out the infographic below for additional information on these outcomes.
An illustration of the outcomes studied in the PUSH-AHF trial.
Statistics
Primary, secondary, safety, and exploratory analyses were performed in the intention-to-treat population. P-value was <0.025 for primary and secondary outcomes and <0.05 for safety and renal endpoints. A p-value of 0.025 was used with Bonferroni correction - a statistical adjustment used to address multiple comparisons in a research study. This correction method mitigates the risk of a Type I error by adjusting the significance level for each test. The Bonferroni-corrected significance level is determined by dividing the original level (0.05) by the number of tests. Given that this study had two co-primary endpoints, the corrected level of significance was set at 0.025.
For 24-hour natriuresis on day 1, each group needed 125 patients to achieve 80% power at a 0.025 significance level. For the second primary endpoint (all-cause mortality or HF rehospitalization at 180 days), 140 patients per arm were needed for 81% power at 0.025 significance, assuming a 38% event rate in the SOC arm, and to detect a hazard ratio of 0.49.
The total 24-hour natriuresis was presented as mean ± standard deviation. Group differences were assessed using the Student's t-test. The impact of natriuresis-guided treatment on long-term outcomes was analyzed with Cox regression for group comparison. Kaplan-Meier estimates were calculated and graphically presented.
Additionally, subgroup analyses were conducted based on age, sex, LVEF, NT-proBNP, eGFR, HF etiology, outpatient diuretic use, sodium and potassium levels, presence of atrial fibrillation, SGLT2 inhibitor use, and new-onset HF. Kaplan-Meier estimates were derived from this data.
The trial did not include a safety monitoring board due to its low-risk nature.
Funding
The study was funded by the Dutch Heart Foundation grant. The study's funder had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Results
Study flowchart of a patients; Figure 1 from Ter Maaten JM et al., Nat Med. 2023.
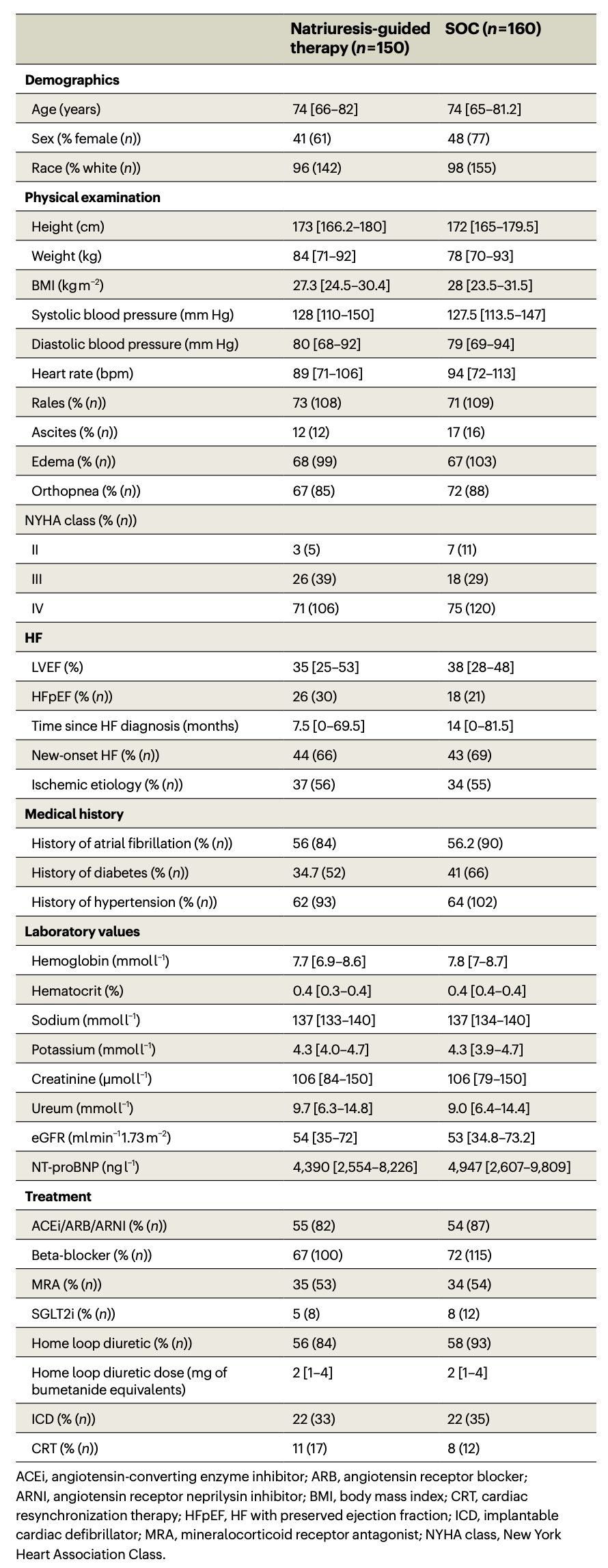
There were 376 eligible patients, 66 were subsequently excluded. No patients switched between arms, and only 1 patient in the control group was lost to follow-up. Of those, 310 patients were randomized: 150 (48.4%) to the natriuresis-guided (NG) diuretic treatment and 160 (51.6%) to the standard of care group (SOC). The median age was 74 years, 45% were female, and the median NT-proBNP was 4710 (2,533 – 8,750).
Baseline clinical characteristics; Table 1 from Ter Maaten JM et al., Nat Med. 2023.
Decongestive treatment
The median IV bumetanide dose at the beginning of the trial was 4 (2-8mg) in both groups. The total dose was higher in the natriuresis-guided treatment group: 26 mg [15.5-44] compared with SOC 15 mg(8.5-32)(P=0.0001). Loop diuretic dose was higher in the natriuresis-guided group at 24 hours and 36 h hours of hospitalization.
The urinary sodium and diuresis-based treatment protocol in PUSH-AHF; Figure 2 from Ter Maaten JM et al., Nat Med. 2023.
According to the pre-specified protocol, the treatment was intensified in 128/150 patients (85%) in natriuresis guided treatment group, during the first 36 hours. An insufficient spot urinary sodium was the reason for intensifying treatment in 100% of patients.
Intensification of treatment during the first 36 hours in the natriuresis-guided group; Extended Data Figure 1 from Ter Maaten JM et al., Nat Med. 2023.
Primary endpoints
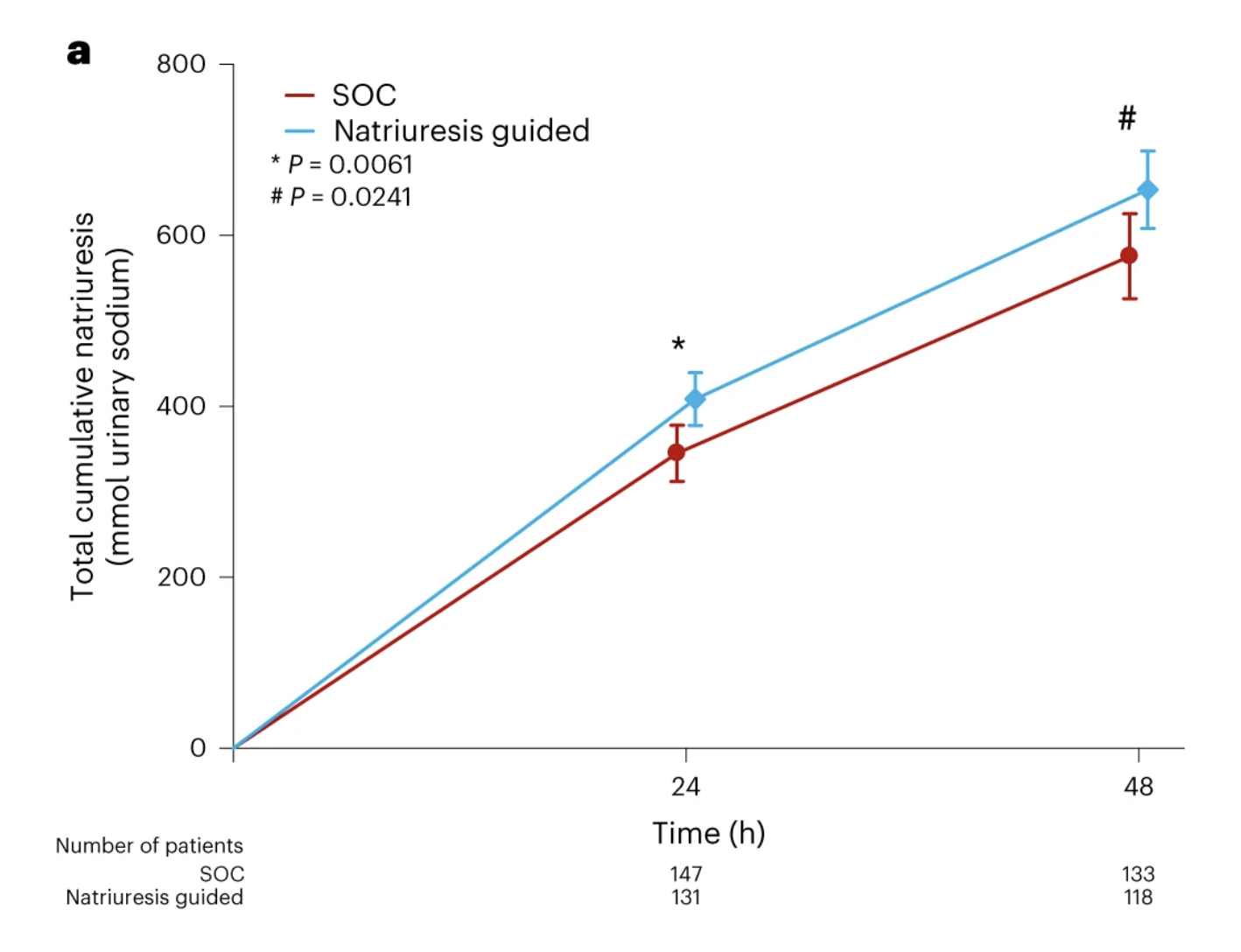
The mean 24-hour natriuresis was greater in the NG group (409 ± 178) compared with the SOC group (345 ± 202) mmol.
All-cause mortality or heart failure rehospitalization at 180 days were not different between groups: 31% of patients in the NG group vs. 31% in SOC group.
There was no difference in all-cause mortality or HF rehospitalization when analyzed as separate endpoints.
Kaplan–Meier plot for the combined primary endpoint of all-cause mortality and HF rehospitalization at 180 days, Cox regression; Figure 3b from Ter Maaten JM et al., Nat Med. 2023.
Secondary endpoints
At 48 hours, mean natriuresis was also greater in the NG group (653 ± 249) compared with the SOC group (575 ± 290) mmol/L.
At 24 hours, mean diuresis was greater in NG (3900) versus SOC (3330) ml.
Natriuresis. Figure 3a from Ter Maaten JM et al., Nat Med. 2023.
Diuresis. Figure 3c from Ter Maaten JM et al., Nat Med. 2023.
Kaplan Meier plot for heart failure rehospitalization at 180 days, Extended Data Fig. 5 from Ter Maaten JM et al., Nat Med. 2023.
Safety analysis
Serious adverse events were similar between groups, with 60 in the natriuresis group versus 70 in the SOC group (p= 0.5799). There were no differences in the doubling of serum creatinine in patients at 24 and 48 hours (0% in the NG group vs. 1% in the SOC group). The incidence of hypokalemia was also similar, with 35% in the NG versus 24% SOC group (p= 0.0849). Serious adverse events at 180 days of follow-up were similar in both groups.
Exploratory endpoints
The differences in natriuresis and diuresis were not sustained at the 72-hour measurements after initiation of protocol-driven diuretic use. The net fluid loss was only statistically greater in the natriuresis-guided group at 24 and 48 hours. There were no statistically significant differences in weight change from baseline, or percent change in BNP at any time during the study.
Safety and exploratory endpoints; Table 3 from Ter Maaten JM et al., Nat Med. 2023.
Discussion
This study was the first randomized controlled trial (RCT) that showed the safety and efficacy of using a protocolized natriuresis-guided diuretic regimen in managing acute heart failure. The primary endpoint of 24-hour natriuresis favored the NG group; however, the second primary endpoint of all-cause mortality and heart failure rehospitalization at 180 days were similar. The protocolized natriuresis-guided diuretic treatment was continued for up to 36 hours, achieving a significant difference in sodium and volume diuresis between the two groups, which unfortunately did not persist beyond 48 hours.
This was a well-done pragmatic RCT that ensured the enrollment of a representative sample of patients with acute heart failure in need of IV diuresis. However, the open nature of this study might put in question the possibility of a Hawthorne effect (i.e., changes in behavior due to observation). Even though the standard of care (SOC) group could intensify diuretic use at the treating physician's discretion, there was a distinct difference in the amounts of diuretics received by patients (total IV Bumetanide: 26 mg in NG vs. 15 mg SOC). The amount of loop diuretic given was higher than in other trials of a similar nature (ENACT and ADVOR), and was reflected in the difference of achieved natriuresis, which also exceeded the natriuresis reported in previous trials. In ADVOR, the average furosemide dose was only 40-80 mg in both arms. This suggests that the natriuresis-guided therapy did increase diuretic dosing, but may have also encouraged (through the inability to completely blind the study due to protocol) increased diuretic dosing in the SOC patients as well.
Major outcomes for studies about heart failure have almost always included all-cause mortality. This trial was no different, but was probably a little too ambitious for such a short intervention. The protocolized natriuresis-guided diuretic treatment was focused on a timed spot urinary sample collection that lasted for a mere 36 hours, and a persistent effect was observed for no more than 48 hours. A short-duration intervention would not be expected to result in major changes in mortality outcomes. Moreover, the prespecified event rate was estimated to be 38%, while the actual event rate was 31%. This points towards an underpowered study, even though the event rate was similar in both groups. Nonetheless, this study can still be considered a win for using spot urinary sodium in guiding acute heart failure diuretic management. It was shown to be an effective, inexpensive, and reliable measure of natriuresis for a sodium avid state, and net-negative sodium balance may still be an important treatment goal. It is still, however, not entirely clear that this strategy is superior to others (i.e., POCUS).
The DOSE trial (Felker GM et al, NEJM 2011) previously reported that patients with acute heart failure receiving higher diuretic doses (IV furosemide at 2.5 times home oral dose) experienced better symptom improvement at the expense of transient worsening of kidney function. PUSH-AHF was associated with a low number of renal safety events, including doubling of serum creatinine in only three patients at 48 hours, despite the high diuretic doses, level of natriuresis, and degree of decongestion. This suggests patients benefit from an individualized treatment approach to relieve congestion rather than a standard high-diuretic approach. It also suggests that there might still be room for even more aggressive natri-diuresis as most of us use worsening azotemia as another subtle signal of adequate diuresis.
There are several observations about the demographic and use of guideline-directed therapy for heart failure that might make this study less generalizable to other populations. First, the patients were almost entirely white and from a limited geographic distribution in the Netherlands. There was practically no heterogeneity regarding cultural, dietary, or social background. In general people from the Netherlands are considered low risk for cardiovascular events using SCORE2 metrics (SCORE2 work group, European Heart Journal 2021). Next, with 70-75% of patients in the study having NYHA class IV heart disease (EF 35% in NG versus EF 38% in SOC), both the European Society of Cardiology (ESC) and the American Heart Association (AHA) recommend five foundational medications (ACEi/ARNI, Beta-blockers, MRA, SGLT2-inhibitors, and loop diuretics) as the standard of care. The patients in this study had very low numbers being treated with these medications, but particularly low numbers of patients on MRAs and SGLT2 inhibitors. This might have also skewed overall outcomes, including hospitalization and mortality, although their use was similar between groups in this study. Finally, it is worth mentioning that heart failure is more than just sodium and water excess. As previously stated, using an endpoint of hospitalization and mortality may have been more than a little stretch for this treatment protocol. Volume overload results from a complex interaction between the sympathetic nervous system, cytokines, natriuretic peptide, and other hormones (i.e., angiotensin II, ADH). Thus, short-term natriuresis alone does not impact the underlying mechanisms of acute decompensated heart failure. Although addressing volume overload may improve certain aspects of the heart failure cycle, diuresis merely slows (but does not stop) disease recurrence and progression to end-stage heart failure.
Conclusion
This is the first randomized control trial assessing the use of natriuresis as a marker for adequate diuretic effect. Unfortunately, this study only showed a short-lived separation of natri-diuresis. Not surprisingly, there were no long-term observed benefits in rehospitalization or mortality. Targeting natriuresis is still of uncertain advantage in patients with AHF, but this study may be a first step in personalizing diuretic therapy.
Summary prepared by
Ivan Rodriguez, Nephrology Fellow
Aldofo Lopez Mateo Regional Hospital, Mexico
and
Sameen Aamer, PGY-2 Internal Medicine Resident,
Allegheny Health Network, Pittsburgh, PA
NSMC Interns, Class of 2023
Reviewed by Cristina Popa, Brian Rifkin, Husam Alzayer
Header Image created by AI, based on prompts by Evan Zeitler