#NephJC Chat
Tuesday April 23, 2019 at 9 pm Eastern
Wednesday April 24, 2019 at 9 pm Indian Standard Time
Wednesday April 24, 2019 at 8 pm BST, 12 noon Pacific
N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744. [Epub ahead of print]
Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators.
PMID: 30990260 Full text at NEJM (free now since online first, till it appears in an issue)
Other Useful Links:
Introduction
Diabetes is a global epidemic. The number of adults living with diabetes has nearly quadrupled since 1980. An estimated 422 million adults are living with diabetes (WHO global report, 2016). Since 30-40% of people with diabetes ultimately develop diabetic kidney disease we can expect a parallel increase in this complication.
Diverse pharmacotherapies have been investigated to halt the progression of diabetic kidney disease. From UKPDS to CREDENCE, we have indeed come a long way (see Figure 1). The RAS (renin-angiotensin system) antagonists delay the progression of diabetic nephropathy. RAS blockade in diabetic nephropathy may be necessary - but is clearly not sufficient. Despite maximal RAS blockade, proteinuria does not completely regress and a substantial proportion of patients with diabetic nephropathy progress to kidney failure. Intensive glycemic control and intensive BP control likewise do not eliminate progression of kidney disease.
While many anti-hyperglycemic agents have been introduced in the last 2 decades, only a handful of them have effects on altering the course of disease progression. Linagliptin is renoprotective in several animal models, this has not been replicated in human studies. CARMELINA did not demonstrate evidence of renoprotection. For the GLP-1 analogues, the most that we have is a modest effect on albuminuria, but nothing on GFR or outcomes that matter. With a better understanding of anti-oxidant pathways, early studies involving bardoxolone beamed signals of hope, but the glory was short-lived as the subsequent trial suggested its association with increased cardiovascular morbidity.
We have now something to cheer about - the SGLT2 inhibitors. They reduce glycemia by causing glycosuria by blocking the SGLT2 transporters in the proximal tubule. This natriuretic effect may hold the key to their kidney effects - since this mediates the effect on the glomerular hyperfiltration via the tubuloglomerular feedback. See this AJKD blog from 2018 by Anna Burgner for a lucid explanation of how this works.
The first suggestion of their utility was whispered by the EMPA-REG trial where use of empagliflozin decreased risk of doubling of creatinine (44%) and end-stage kidney disease (55%) without any difference in the degree of albuminuria. We covered the renal results on NephJC here - the renal outcomes beyond new onset nephropathy were post hoc outcomes. A similar pretty picture was painted on the CANVAS too where canagliflozin reduced the risk of sustained and adjudicated major kidney outcomes. Discussed here on NephJC, on the basis of the prespecified hypothesis testing sequence the renal outcomes were not viewed as statistically significant. They did show a possible benefit of canagliflozin with respect to the progression of albuminuria (hazard ratio, 0.73) and the composite outcome of a sustained 40% reduction in the estimated GFR, the need for renal-replacement therapy, or death from renal causes (hazard ratio, 0.60). These were mostly driven by the albuminuria and 40% GFR outcome, since the patients in CANVAS and EMPAREG were low risk for progression of CKD.
Table 1: Comparison of the Major RCTs in SGLT2i, pre-CREDENCE
Another wrinkle in the story is the signal of higher amputations reported in CANVAS - and then reinforced by observational data from elsewhere.
The results of CREDENCE trial have been welcomed by the world with a thunderous applause. This trial (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) was designed to assess the effects of canagliflozin on primarily the renal outcomes in patients with established nephropathy. To note, CREDENCE was initiated in 2014, before the results of EMPAREG came out. Another point is that there was a suggestion that the efficacy of these drugs decreased with decreasing GFR - and the previous trials mostly enrolled patients with preserved GFR. This makes the enrolment and outcomes of CREDENCE all the more important to understand.
The Study
Methods
This was a randomized, double-blind, placebo-controlled trial conducted at 690 sites in 34 countries across North America, Latin America, South Africa and Asia Pacific.
Inclusion criteria
Age ≥ 30 years
Type 2 Diabetes mellitus with an HbA1C ≥ 6.5% and ≤12.0% (≤ 10.5% in Germany)
Estimated GFR 30 - 60 ml/min/1.73m^2 (using CKD-EPI equation)
Patients needed to be on a stable maximum tolerated daily dose of ACEi or ARB for at least 4 weeks prior to randomisation (however dual RAS blockade and MRAs/DRIs were not allowed).
Albuminuria defined by urine albumin to creatinine ratio (UACR) of 300 – 5000 mg/g.
Because the trial was designed to study the impact of Canagliflozin on the progression of CKD, the intent was that at least 60% of the patient population have CKD stage 3 (rather than stage 2), with an eGFR of 30-60 ml/min/1.73m2.
Exclusion criteria
Type 1 Diabetes mellitus
History of diabetic ketoacidosis
History of hereditary glucose-galactose malabsorption or primary renal glycosuria
Renal disease that required treatment with immunosuppressive therapy
Known significant liver disease
Current or history of NYHA Class IV heart failure
Blood potassium level > 5.5 mmol/L at the time of screening
What was done next…
Patients who met the eligibility criteria were enrolled in a 2-week, single-blind placebo-run in period. Patients who failed to take ≥80% of the scheduled run-in treatment were deemed ineligible.
Eligible patients were randomized (1:1) to receive either Canagliflozin (100mg orally once daily) or matching placebo. Randomization was stratified according to the category of estimated GFR at the time of screening
30 to <45 ml/min/1.73m2.
45 to <60 ml/min/1.73m2.
60 to <90 ml/min/1.73m2
Background care
Glycemic control was reinforced with diet, exercise counselling and also as per the discretion of the responsible physician.
Patients were followed up at 3, 13 and 26 weeks, then alternated between telephone calls and out-patient visits every 13 weeks.
Figure 2 from Jardine MJ et al, American Journal of Nephrology, 2017
Primary outcome
Composite of
End-stage kidney disease (defined as dialysis for at least 30 days) or an eGFR of <15ml/min/1.73m^2 sustained for at least 30 days
Doubling of serum creatinine
Death from renal or cardiovascular disease
Secondary outcomes
Secondary end points were analysed in a pre-specified hierarchical order
1st - composite of cardiovascular death or hospitalisation for heart failure
2nd- composite of cardiovascular death, MI, or stroke
3rd - hospitalisation for heart failure
4th- composite of ESKD, doubling of s.creatinine or renal death
5th- cardiovascular death
6th- death from any cause
7th- composite of cardiovascular death, MI, stroke or hospitalisation for heart failure or unstable angina
Heirarchical analysis meant that statistical significance was required before testing the next hypothesis in the sequence mentioned.
Statistics simplified
The trial was event-driven – meaning that the study was driven by the occurrence of the primary outcome rather than being of fixed observation time. Idea was to detect a 20% risk reduction in the primary end-point with 90% power. Why was a figure of “20% risk reduction” chosen? – that’s because a 20% relative risk reduction was considered clinically meaningful, commensurate with the similar reduction seen in trials involving RAS blockade, namely, RENAAL and IDNT trials. This required enrolment of at least 4200 patients (844 events).
Prespecified stopping guidance that was provided to the data monitoring committee by the steering committee proposed possible recommendation of early cessation if clear evidence of benefit was observed for the primary outcome (P<0.01) and the composite of end-stage kidney disease or death from renal or cardiovascular causes (P<0.025), with consideration of the overall balance of risks and benefits.
Funding
Funded by Janssen Research and Development as a collaboration between the sponsor, an academic-led steering committee, and an academic research organization, George Clinical. Members of the steering committee designed the trial, supervised its conduct, and were responsible for reporting the results. Analyses were performed by the sponsor and independently confirmed at George Clinical with the use of original data. The first draft of the manuscript was drafted by the first and last author.
Results
Figure S1 from Perkovic et al, NEJM, 2019
Table 1 depicts the baseline demographic characteristics of patients. This was a population of patients that is very familiar to us: all diabetic nephropathy, duration of diabetes ~ 15.5 years. About two-thirds were on insulin and over half on metformin. Despite the 30 ml/min GFR floor, about 170 participants with GFR 15-30 snuck in (see table S1). Of more interest to us, 10% had nephrotic range proteinuria and another three-quarters had non-nephrotic, but macroalbuminuria.
Table 1 from Perkovic et al, NEJM, 2019
Table S2 from Perkovic et al, NEJM 2019
Excerpt from Table S1, Perkovic et al, NEJM, 2019
Primary outcomes
Instead of 844 outcomes, the DSMB recommended the trial be halted based on the stopping rules mentioned above, after the interim analyses. By the time all events were adjudicated and recorded, ~ 500 primary outcome events had occured.
There was a 30% relative risk reduction in the primary composite end-point of ESKD, doubling of serum creatinine and renal or cardiovascular death, with line segments diverging as early as 12 months after randomization (event rate – 43.2 vs 61.2 per 1000 patient-years respectively in canagliflozin vs placebo arms).
If we dissect the primary outcomes into individual components, the relative risks of ESKD, doubling of serum creatinine and death due to cardiovascular cause were reduced by 32%, 40% and 22% respectively.
Figure 1 from Perkovic et al, NEJM 2019
On subgroup analysis, with respect to relative risk reduction in the primary outcomes, the effect was consistent across all the subgroups - as shown below. Pay attention to the interaction p values (last column) in the figures below.
Figure 2 from Perkovic et al, NEJM 2019
Figure S3 from Perkovic et al, NEJM 2019
Secondary outcomes
Patients in to the canagliflozin arm had a lower risk of most secondary outcomes (statistically significant for secondary outcomes listed as 1st to 4th). However, there was no significant difference in the risk of cardiovascular death, so the subsequently listed outcomes in the hierarchy were not formally evaluated.
The significantly lower rates of cardiovascular events in CREDENCE mirror the results of CANVAS, EMPA-REG OUTCOME and DECLARE-TIMI 58 trials.
Canagliflozin group fared better in terms of the degree of albuminuria too, being 31% lower as compared to placebo. The difference appears almost immediately as depicted in the figure… suggesting an early hemodynamic mechanism probably mediated by reduced intraglomerular pressure.
Figure 3a from Perkovic et al, NEJM 2019
This may be supported by early GFR decline seen in the first few weeks of initiation of Canagliflozin. But thereafter, the slope of decline of eGFR remains gentler for Canagliflozin, for a difference of 2.74 ml/min/1.73m^2.
Figure 3b from Perkovic et al, NEJM 2019
Could these outcomes be due to the effect on BP and blood sugar?
Probably not. Let’s see why..
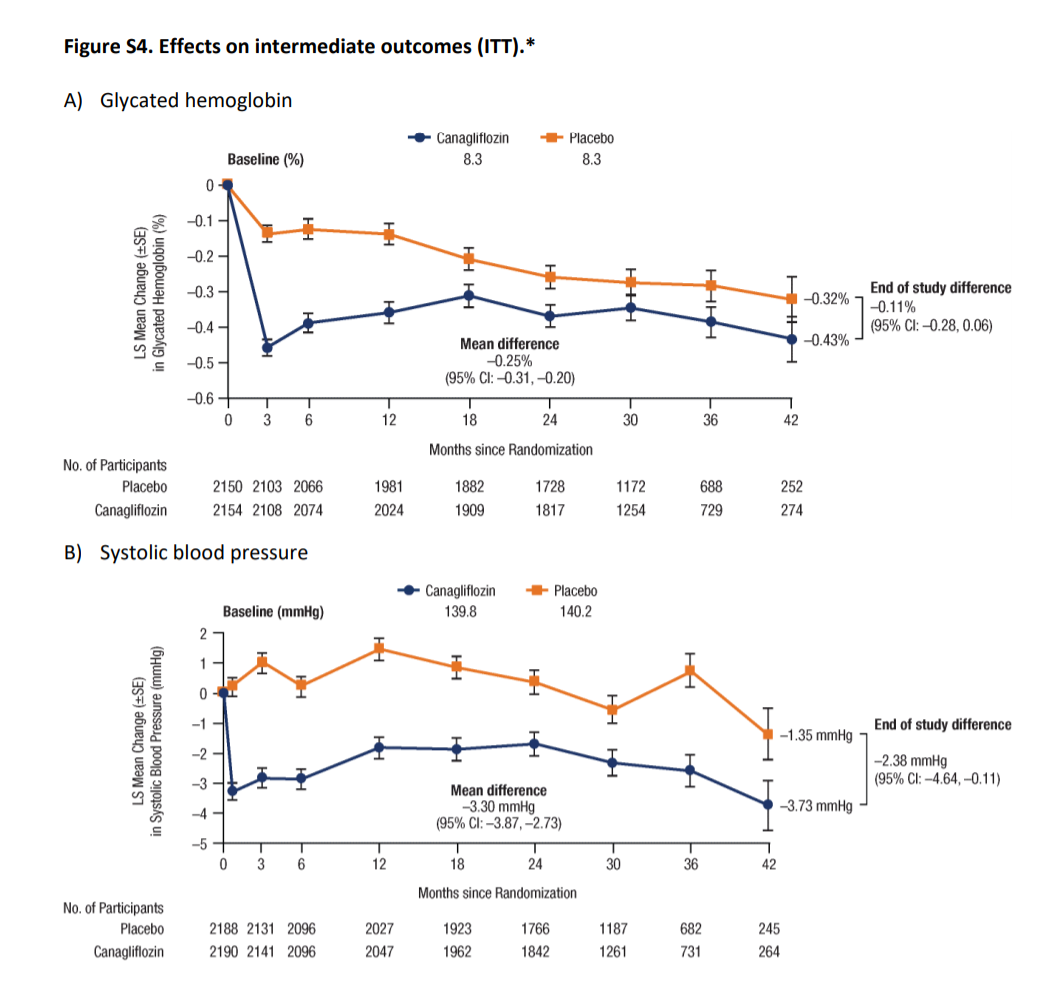
Figure S4 from Perkovic et al, NEJM 2019
Glycemic control:
Baseline mean HbA1C across both the groups was 8.3%. While the mean levels were lower in Canagliflozin arm the difference was marginal (mean being -0.25% and end of study difference being just -0.11%). As seen from the table S2 above, this occurred despite insulin and other hypoglycemic use being higher in the placebo group. However, at these levels of glycemia, we don’t have any data to support an outcome difference, especially with such a small change.
BP control
The mean difference in systolic and diastolic BP were 3.30 and 0.95 mmHg, lower in the Canagliflozin arm. Again, this is too small a difference to result in such a large nephroprotective effect.
Body weight
Patients in the Canagliflozin arm were lighter by approximately 0.80 kg compared to the placebo in spite of similar BMI at screening; the difference was maintained along the entire duration of the study period. Not an unwelcome effect to have.
Safety
Lower limb amputation – the rates were similar across both the groups and so were the fracture rates. This is a stark difference from the CANVAS results, which raised the issue in the first place. In CREDENCE, a protocol amendment in May 2016 asked investigators to examine patients’ feet at each trial visit and temporarily interrupt the assigned treatment in patients with any active condition that might lead to amputation. Is this all that is needed to prevent amputations?
Overall the urinary tract infections occurred at similar frequencies in both the arms (48 vs 45 episodes per 1000 patient-years). However, genital mycotic infections occurred more commonly in patients receiving canagliflozin.
Ketoacidosis was detected with greater frequency in the canagliflozin arm than the placebo. All but one patient had concomitant blood glucose levels more than 250 mg/dl suggesting a low risk of so-called euglycemic ketoacidosis.
Risk factors for ketoacidosis included (see table S6 for more details)
Background insulin therapy
Longer duration of diabetes
HbA1C > 10%
Discussion
So yes, we all have good reasons to go ga-ga over this molecule. With renal and cardiovascular benefits, these drugs can gift quality and quantity of life to patients living with diabetes.
To reiterate the strengths of the study, it must be emphasized that this one of those few studies, where the renal outcomes were given prime importance. The benefit was obtained over and above the RAS blockade therapy. As with any study, CREDENCE has its own limitations. As the authors agree premature termination of the trial limited the power for at least some secondary outcomes. Secondly, those with very advanced kidney disease (GFR < 30) were excluded from the study, though a few patients with GFR 15-30 did make it in. This is more a question of generalizability - and to consider at what GFR (if any) would one not use, or stop an SGLT2i.
What else is CREDENCE bringing to the world?
A summary of some future SGLT2i trials. Which ones are you excited about?
Table 2: Ongoing trials of SGLT2i of interest to the Nephrology Community
In addition to these - we also have a few heart failure trials (DAPA-HF followed by the EMPEROR and EMPERIAL trials) which include diabetes as well as non-diabetic patients, go down to as low a GFR as 20, and have prespecified kidney endpoints.
Summary prepared by: Lovy Gaur
Nephrologist
NSMC Intern 2019