#NephJC Chat
Tuesday April 9th 9pm Eastern
Wednesday April 10th 9pm IST
Wednesday April 10th 8pm GMT, 12 noon Pacific
Ann Intern Med. 2019 Feb 26. doi: 10.7326/M18-2229. [Epub ahead of print]
Comparative Efficacy of Therapies for Treatment of Depression for Patients UndergoingMaintenance Hemodialysis: A Randomized Clinical Trial.
Mehrotra R, Cukor D, Unruh M, Rue T, Heagerty P, Cohen SD, Dember LM, Diaz-Linhart Y, Dubovsky A, Greene T, Grote N, Kutner N, Trivedi MH, Quinn DK, Ver Halen N, Weisbord SD, Young BA, Kimmel PL, Hedayati SS.
PMID: 30802897
Introduction
Depression is widely recognized as the most common mental health condition among patients on maintenance hemodialysis (HD), affecting about 25%, a rate that is over four-fold higher than in the general population . Patients on HD face several unique physical and psychological stresses that contribute to this, including comorbidities, dietary restrictions, high pill burden, and the need to attend for prolonged thrice-weekly treatment. These factors contribute to a decrement in both physical and psychological well-being. The presence of depression is associated with lower quality of life, increased hospitalizations, non-adherence with treatment including shortened treatments and skipping dialysis treatments, and decreased adherence with fluid restriction.
Unfortunately, depression is under recognized and under treated. Patients are often reluctant to accept the diagnosis of depression and are also reluctant to accept treatment. There are additional challenges that must be considered in the HD population:
When we do recognise depression in dialysis patients?
What do we do next?
Treatments include cognitive behavioral therapy (CBT) which helps patients with behavioral modification strategies and offers problem solving skills, and antidepressant medications including selective serotonin re-uptake inhibitors (SSRI). However, evidence for the efficacy of antidepressant therapies in this population is limited.
The CAST Randomized Clinical Trial was published in 2017 and included 201 patients with non-dialysis dependent chronic kidney disease and at least moderate depressive symptoms. The use of sertraline vs placebo did not result in a statistically significant difference in symptom improvement over 12 weeks (−4.1 points vs −4.2 points on the 16-item Quick Inventory of Depression Symptomatology).
The ASSertID trial was a randomized, double-blind, placebo-controlled trial of sertraline over 6 months in patients on hemodialysis with depression to determine study feasibility, safety, and effectiveness. They found a high proportion of depresssion (32.3%) as diagnosed by the Beck Depression Index Score. They found no significant differences between sertraline and placebo groups however the authors highlighted several issues including a high drop-out rate and recruitment issues. One problem was that a lot of patients were already on antidepressants and so declined to participate.
Patients on HD have unique risk factors that affect the risk-benefit equation for both safety of pharmacological treatment and acceptance of CBT. Safety is a particular concern, especially from a cardiovascular perspective given the high prevelance of cardiovascular disease in this patient group, the risk of interaction with other medications, and electrolyte shifts on HD. A study in the April 2019 issue of JASN sought to address this. Check out the VA by @Stones_ below. They found an increased risk of sudden cardiac death among HD patients receiving SSRIs with higher potential to prolong QT interval (citalopram and escitalopram) versus lower (fluoxetine, fluvoxamine, paroxetine, sertraline) . This risk was enhanced among elderly females, those with pre-existing conduction disorders, and those taking other QT-prolonging medications.
So far, we know that depression is common in CKD patients, and it is hard to treat - and hard to do trials in this population. Pharmacotherpay that works in the general population carries risks, and may not be effective. Can we ascend from the depths of this depressing set of facts?
The Study
Methods
ASCEND, A Trial of Sertraline vs. CBT for End-stage Renal Disease Patients with Depression was designed to inform care of patients with a major depressive disorder undergoing HD. This was an was an open-label, parallel-group, multicenter, randomized controlled trial.
The aim was to determine the effect of an engagement interview on treatment acceptance (Phase 1) and to compare the efficacy of CBT versus sertraline (Phase 2) for treating depression in patients on HD. Participants were randomised 1:1 for both phases.
Participants were enrolled between March 2015 and August 2017 and were followed through November 2017. They were receiving in-centre hemodialysis at 41 dialysis facilities at 3 clinical sites—1 each in Albuquerque, New Mexico; Dallas, Texas; and Seattle, Washington—operated by 6 dialysis providers.
Phase 1:
This involved engagement interview aimed toward increasing participants’ willingness to accept the diagnosis and treatment; face to face intervention by trained therapists while patients received outpatient HD. Used motivational interviewing, participants were also given a 20 minute DVD to improve their understanding of depression and it’s treatment.
Primary outcome was the proportion of patients initiating treatment for depression either inside or outside of the trial within 28 days of the intervention
Secondary outcome was the percentage willing to accept treatment within 14 days
Phase 2:
Participants assigned to the CBT group were scheduled for 10 sessions of 60 minutes each over 12 weeks while they received outpatient HD. The sessions were conducted face-to-face by 5 therapists; each patient saw the same therapist for all 10 sessions. Patients self-reported their depressive symptoms by using the Quick Inventory of Depressive Symptoms–Self-Report (QIDS-SR) every 2 weeks for the first 6 weeks and every 3 weeks for the next 6 weeks
Participants in the sertraline group had their dose titrated every 2 weeks for the first 6 weeks and then maintained for 6 weeks. Depression severity was assessed with the QIDS-SR and drug tolerability with the Frequency, Intensity, and Burden of Side Effects Ratings scale. Sertraline therapy was started with 25 mg/d during the first week and increased to 50 mg/d in the second week with a goal dose of 200mg, unless limited by adverse effects.
Primary outcome was the 16-item Quick Inventory of Depressive Symptoms–Clinician Rated (QIDS-C) score at 12 weeks.
Secondary outcomes were patient-reported outcomes at 12 weeks; computer-assisted telephone interviewing and measures of adherence
Statistical Analysis 180 randomly assigned patients would provide 80% power with a 2-sided of 0.05 to detect a difference in the mean 12-week QIDS-C score of between 0.327 (R = 0.70) and 0.419 (R = 0.40) of 1 SD.
Funding source: The trial was funded by PCORI, which had no role in the conduct, analysis or interpretation.
Results
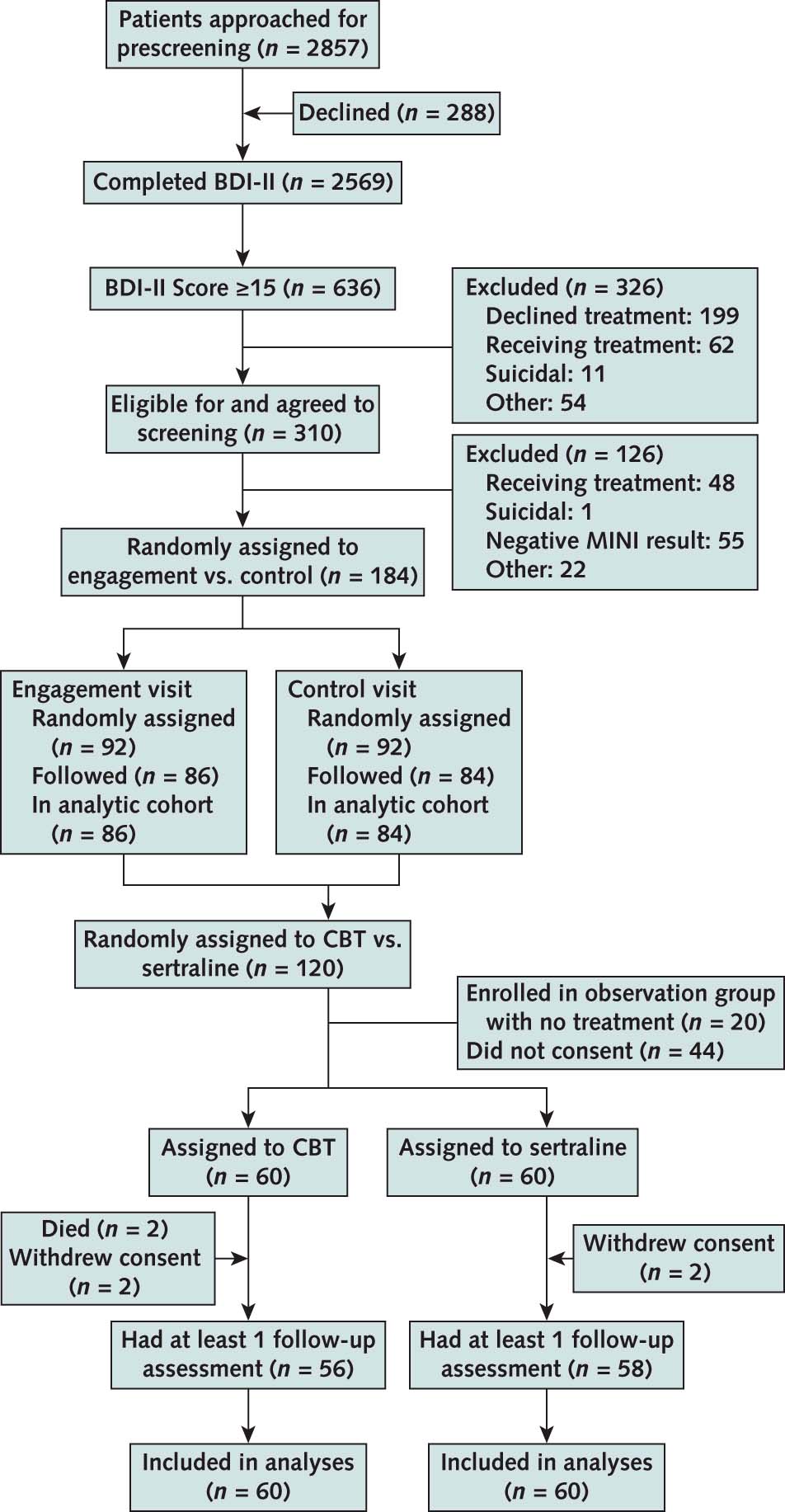
Of the 184 participants enrolled in Phase 1, 179 (97%) completed the study visit (90 in the engagement and 89 in the control group). The engagement interview and control visit lasted 53.3 (SD, 16.6) and 30.3 (SD, 15.1) minutes, respectively.
Figure 1 from Mehrotra et al, Annals 2019
Of the 60 patients randomly assigned to the CBT group, 2 died and 2 withdrew consent. The participants completed a median of 5 sessions by 6 weeks and 10 sessions by 12 weeks; 80% completed 8 and 73% completed 10 sessions in 12 weeks. Of the 60 patients randomly assigned to the sertraline group, 2 withdrew consent. At weeks 6 and 12, a total of 78% of the participants were receiving sertraline. The median prescribed dose of sertraline at 6 and 9 weeks was 150 mg, with 55% and 49%, respectively, receiving a dose of 150 mg or higher.
Primary End Points
Phase 1: The primary outcome was assessed for 170 (92%) of participants (86 engagement and 84 control group). The proportion of participants who accepted treatment for depression within 28 days did not differ between the engagement and control groups (66% vs. 64%, respectively; P = 0.77; estimated risk difference, 2.1% [95% CI, 12.1% to 16.4%]).
Phase 2: QIDS-C scores decreased in both groups; CBT baseline, 12.2 [SD, 5.1]; 12 weeks, 8.1 [SD, 5.1]), sertraline baseline, 10.9 [SD, 4.9]; 12 weeks, 5.9 [SD, 4.5]. Depression scores at 12 weeks were lower in the sertraline than the CBT group (P =0.035). The proportion of patients with a decrease in QIDS-C score of at least 50% or a QIDS-C score less than 5 at 12 weeks was greater in the sertraline group; however estimates were imprecise and had wide CIs (≥50% decrease in score: 36% in the CBT and 43% in the sertraline group; rate ratio, 1.18 [CI, 0.75 to 1.87]. No changes in QIDS-C score were observed among the participants who declined treatment within or outside the study.
Secondary End Points
Phase 1: Between the engagement and the control group, no difference was found in the proportion of patients intending to start treatment for depression 2 weeks after the visit (81% vs. 81%; P = 0.96; estimated risk difference, 0.3% [CI, 11.2% to 11.8%]).
Phase 2: At 12 weeks, 5 of 9 patient reported outcome measures (BDI-II, Sheehan Disability Scale, energy/vitality subscale of Short Form-36, Satisfaction With Life Scale, and Pittsburgh Sleep Quality Index) were better for the sertraline than the CBT group.
Adverse Events:
Serious adverse events occurred in both groups: 13 events in 11 patients in the CBT group, 18 events in 14 patients in the sertraline group. “Non-serious” adverse events were more frequent in the sertraline (56 events in 25 patients) than the CBT (17 events in 12 patients) group.
Discussion
The authors cite 3 key findings from this study:
Engagement interview had no effect on patients’ acceptance of depression diagnosis or treatment
Depressive symptoms and other patient-reported outcomes improved in both the sertraline and CBT groups; outcome scores were modestly better in the sertraline group
Adverse events occurred more frequently in the sertraline group than the CBT group
Strengths of this study include a diverse patient population, high adherence to assigned intervention and close follow up period. Limitations include a lack of a “no treatment” control group, the comparative effect of 2 treatments on secondary patient-reported outcomes was attenuated toward the null on multiple imputation and short follow up period.
In conclusion, recognition, diagnosis and treatment of depression in the dialysis population remains a clinical challenge.
Summary by Laura Slattery