#NephJC Chat
Tuesday Feb 11 9 pm Eastern
Wednesday Feb 12 9 pm IST
Wednesday Feb 12 9 pm GMT
J Am Soc Nephrol. 2020 Feb;31(2):350-364. doi: 10.1681/ASN.2019060618. Epub 2019 Dec 26.Paperpile
Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN.
Antonelou M, Michaëlsson E, Evans RDR, Wang CJ, Henderson SR, Walker LSK, Unwin RJ, Salama AD; RAVE-ITN Investigators.
PMID: 31879336 Full Text at JASN
Introduction
Background on ANCA-associated glomerulonephritis.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis is the most common form of new-onset glomerulonephritis in patients over age 50, although it can occur at any age. It is characterized by a necrotizing vasculitis which affects small blood vessels, which include capillaries (including glomerular and pulmonary vascular bed capillaries), venules, arterioles, and small arteries by injury to endothelial cells. ANCAs are specific for either myeloperoxidase (MPO) or proteinase 3 (PR3), with a subset of patients having antibodies directed against both. The ANCA disease spectrum includes microscopic polyangiitis, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and renal limited vasculitis.
Fibrocellular crescent from a case of ANCA-associated glomerulonephritis
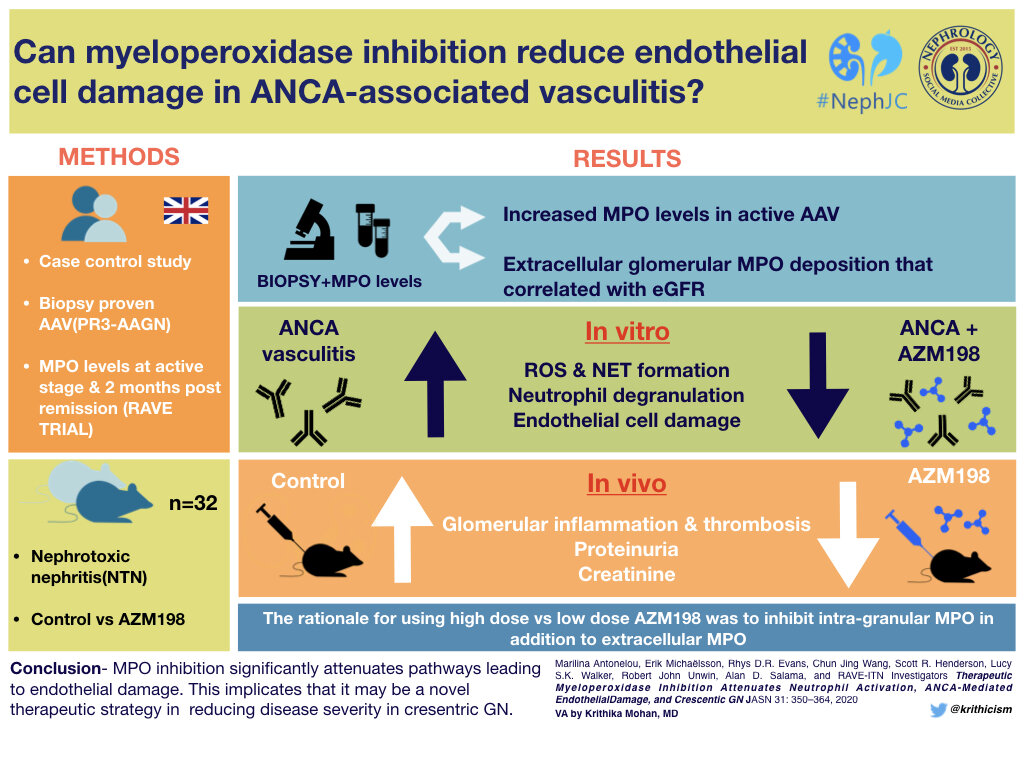
Current therapies for ANCA-associated vasculitis / glomerulonephritis include strong immunosuppression, usually with cyclophosphamide and glucocorticoids. Rituximab and plasmapheresis are additional therapies to reduce production of and remove pathogenic antibodies. A clinical trial evaluating use of plasma exchange and glucocorticoids for the treatment of ANCA-associated vasculitis (PEXIVAS) is completed, and will be published in the NEJM on Feb 13. The requirement for chronic glucocorticoid use is associated with complications, and therefore new treatment targets are needed. In the current study, Antonelou et al evaluated a novel treatment, myeloperoxidase inhibition, as a treatment target in ANCA-associated glomerulonephritis. Let’s examine the role of MPO in ANCA-associated GN, and how this would be feasible to target in this disease.
Biology of ANCA-associated glomerulonephritis.
In ANCA-associated crescentic glomerulonephritis, neutrophil and monocyte activation and production of reactive oxygen species leads to increased tissue damage. Upon neutrophil activation, myeloperoxidase (a heme-containing peroxidase) is released from phagolysosomes inside neutrophils, promoting degranulation of neutrophils in response to innate immune cytokines. This is activated by hydrogen peroxide and leads to production of multiple reactive oxygen intermediates, which oxidize and chlorinate multiple cellular components (including proteins, lipids, and DNA).
Myeloperoxidase (MPO) is involved in the innate immune defense and is critical to the formation of neutrophil extracellular traps (NETs). Reactive oxygen intermediates stimulate MPO to trigger activation and translocation of neutrophil elastase from granules to the nucleus, which leads to decondensation of chromatin by acting on histone proteins. Formation and clearance of neutrophil extracellular traps is regulated by a process known as NETosis. NETosis is implicated in autoimmunity in ANCA-associated glomerulonephritis, as MPO within NETs promotes antigen-specific T and B cell activation, and NETs contain antigens that can be recognized by ANCAs to amplify this response. Extracellular deposition of myeloperoxidase (MPO) results in antigen-specific lymphocyte activation (involving both T and B cells) and increases tissue injury. NETs could be the mechanism through which there is a break in tolerance to ANCA autoantigens.
Myeloperoxidase-deficient mice have attenuated glomerulonephritis in a model of nephrotoxic nephritis. Therefore, the authors hypothesize that MPO inhibition has the potential to treat crescentic glomerulonephritis. Selective MPO inhibitors have been studied in other models, including vasculitis, heart failure and pulmonary hypertension. The authors utilize a MPO inhibitor, AZM198 in vitro and in vivo in a preclinical proof-of-concept study to determine if there is utility for the treatment of ANCA-associated glomerulonephritis. This compound is membrane permeable and is a substrate for MPO that inhibits extracellular MPO at low dose, and intragranular MPO within neutrophils at high dose.
Methods to examine the effects of MPO inhibition in ANCA-associated GN.
The authors included in vitro studies from patients with ANCA-associated glomerulonephritis and in vivo studies in two mouse models of nephrotoxic nephritis.
Studies in patients with crescentic glomerulonephritis:
Evaluation of renal biopsies from patients, including MPO-ANCA (n=5), PR3-ANCA (n=3), IgA nephropathy (n=4), lupus nephritis (n=4), ANCA-negative crescentic GN (n=2), as well as CKD and healthy controls.
(a) Distribution of MPO positive cells, by immunohistochemistry (IHC).
(b) Endocapillary hypercellularity / neutrophilic infiltration within glomerular capillaries (CD15+ cells by IHC)
Serum testing / in vitro studies from patients with crescentic glomerulonephritis:
Serum was tested from healthy controls and patients enrolled in the Rituximab in ANCA-associated Vasculitis (RAVE) trial.
Concentration of MPO and human neutrophil peptides.
Formation of neutrophil extracellular traps
Production of reactive oxygen species
Neutrophil degranulation studies
Neutrophils were activated by the phorbol 12-myristate 13-acetate (PMA) and TNF-alpha, followed by exposure to IgG from healthy controls or ANCA patients (after concentrating on protein G columns).
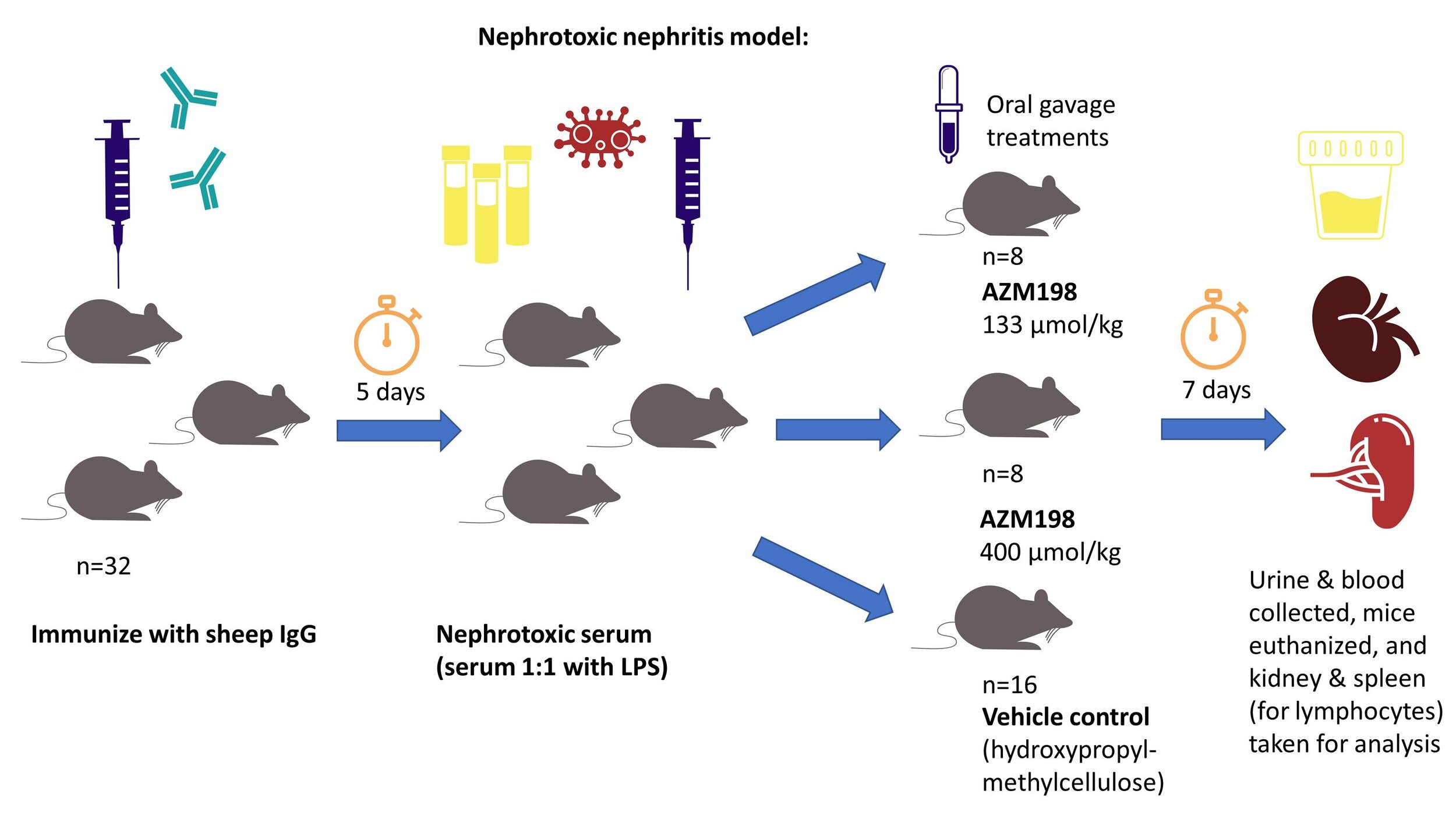
Murine model of nephrotoxic nephritis:
The MPO inhibitor AZM198 was tested in a mouse model of nephrotoxic nephritis. In this model, mice were immunized with sheep immunoglobulin, treated with LPS-containing sera 5 days later, and then randomized to receive a vehicle control or AZM198 (at low dose or high dose) by oral gavage for 7 days of treatment. Urine and blood samples were taken, and at the end of the study, the animals were sacrificed with the kidney (for histology) and spleen (for lymphocyte studies) were taken for analysis.
An overview of this mouse model is shown below:
This mouse model causes an immune complex-driven glomerulonephritis with augmentation of a Th1 and Th17-mediated adaptive immune response. At the end of the study, FFPE kidney sections were scored for glomerular capillary loop fibrin thrombi. Additionally, MPO and F4/80 staining (to quantify macrophages) was performed by immunofluorescence microscopy. Ly6g (a marker for murine neutrophils) staining on frozen sections was performed to quantify neutrophilic infiltration within glomeruli. Serum was tested for anti-sheep immunoglobulin IgG, against IgG1, IgG2, and IgG3 using ELISAs.
To establish T cell responses, an adoptive transfer model was also studied, utilizing DO11.10 mice, which are transgenic mice containing a specific T cell receptor specific to an ovalbumin peptide. The ovalbumin peptide was injected into the mice (same peptide the transgenic TCRs are directed against) to trigger an immune response, and the mice were then treated with vehicle control or AZM198 by oral gavage. Draining lymph nodes were harvested at the end of the study and stained for CD4, CD44, and the transgenic TCR (DO11.10) to assess by flow cytometry.
Results
Myeloperoxidase activity is increased in patients with ANCA-associated glomerulonephritis, compared to healthy controls and renovascular disease controls (Fig 1A). MPO-containing sera causes neutralization and reduces MPO levels by approximately 40%, therefore some PR3-ANCA patients had increased MPO concentrations detected than MPO-ANCA. MPO concentration falls in most patients along side clinical remission. (Fig 1B).
Figure 1 from Antonelou et al, JASN 2019
Extracellular MPO deposition is present in patients with crescentic glomerulonephritis, including patients with PR3-ANCA (Fig 2A), MPO-ANCA (Fig 2B), ANCA-negative crescentic GN (Fig 2C), and crescentic IgA nephropathy (Fig 2D). Isotype controls worked appropriately (Fig 2E, F, data not shown).
Figure 2A-D from Antonelou et al, JASN 2019
Myeloperoxidase levels correlate with glomerular filtration rate (GFR) and crescents on renal biopsy in patients with ANCA-associated glomerulonephritis (MPO or PR3), ANCA-negative glomerulonephritis, crescentic IgA nephropathy, and proliferative lupus nephritis. Results of whole kidney MPO are seen, with similar results seen with glomerular MPO (Fig 3C and Fig 3D, data not shown) and extraglomerular leukocyte MPO (Fig 3E and Fig 3F, data not shown). Tubulointerstitial MPO deposition correlated with eGFR, but not crescent formation (Supplemental Table 2, data not shown). These data suggest that MPO is an important driver of renal inflammation.
Figure 3A-B from Antonelou et al, JASN 2019
To evaluate the functional effects of MPO inhibition, peroxidase activity, ROS production, human neutrophil peptide (HNP) levels, and formation of NETs were studied. AZM198 reduced formation of reactive oxygen intermediates, as determined by rhodamine 123 fluorescence in DHR-primed neutrophils (Fig 4A), marginally reduced human neutrophil peptide levels (Fig 4B), and reduced formation of neutrophil extracellular traps (Fig 4C) in neutrophils primed with TNF-alpha and stimulated with PR3-ANCA sera.
Figure 4A-C from Antonelou et al, JASN 2019
Here’s what the NETs look like, evaluated by Sytox green staining of permeabilized cells, which binds DNA. NETs were increased in primed neutrophils (stimulated with TNF-alpha) and was reduced in the presence of MPO inhibitor AZM198 (Fig 4F, control shown in 4E, and unprimed neutrophils in 4D).
Figure 4 E-F from Antonelou et al, JASN 2019
The assay for the formation of reactive oxygen species involves loading neutrophils with dihydrorhodamine (DHR), stimulating neutrophils, and measuring rhodamine 123 fluorescence in the FL1/FITC channel by flow cytometry. DHR is not normally fluorescent, but rhodamine 123 is generated in the presence of reactive oxygen intermediates, which does fluorescence. Increased rhodamine 123 fluorescence in DHR-loaded neutrophils occurs when neutrophils are stimulated with immunoglobulin stimulated from ANCA-positive patients, but not healthy controls (supplemental figure 1).
Supplemental Figure 1 from Antonelou et al, JASN 2019
Co-culture studies were performed with neutrophils and human vein endothelial cells (derived from umbilical cord tissue), to evaluate if myeloperoxidase inhibition reduces endothelial cell damage. This was performed as activated endothelial cells can produce neutrophil extracellular traps and lead to endothelial cell death (Gupta et al, 2010). BrdU labeled endothelial cells were primed with TNF-alpha and incubated with ANCA-stimulated neutrophils in the presence/absence of MPO inhibitor AZM198. BrdU incorporates into DNA, and when cell death occurs, is released into the supernatants.AZM198 reduced endothelial cell death (Fig 5A). There was a similar trend, but non-significant decrease in vWF release into the supernatants (Fig 5B). Endothelial cell morphology was altered after priming with ANCA-stimulated neutrophils, and AZM198 prevented / reduced these morphologic changes (Fig 5C-F, data not shown).
Figure 5 A-B from Antonelou et al, JASN 2019
Now on to the in vivo studies . . .
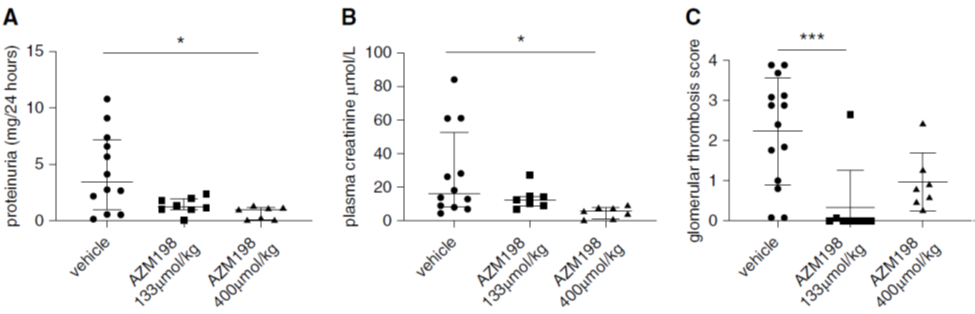
Treatment of mice with MPO inhibitor AZM198 after induction of nephrotoxic nephritis reduced proteinuria (Fig 6A), plasma creatinine (Fig 6B), and glomerular thrombosis (Fig 6C). This occurred at both low dose (133 µmol/kg) and high dose (400 µmol/kg) of the drug.
Figure 6 A-C from Antonelou et al, JASN 2019
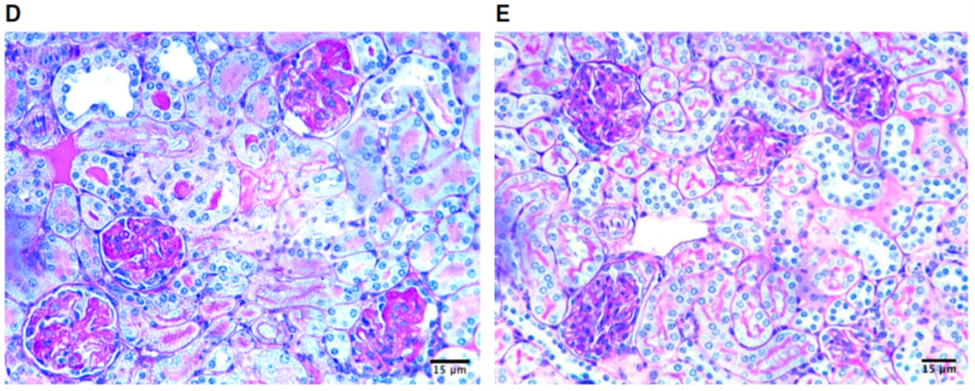
Glomerular capillary loop fibrin thrombi are absent with AZM198 MPO inhibitor (Fig 6E), which were present in the vehicle control (Fig 6D).
Figure 6 D-E from Antonelou et al, JASN 2019
MPO inhibition with AZM198 reduced macrophage infiltration into glomerular capillaries (Fig 7A) and MPO concentration (Fig 7B).
Figure 7 A-B from Antonelou et al, JASN 2019
This corresponded to reduced macrophage and CD4+ T cell infiltration within kidneys, as seen by immunofluorescence staining (reduced macrophage infiltration shown here in Fig 7F, compared to vehicle control Fig 7E). CD4+ T cell infiltration shown the same trend (Fig 7G). Neutrophilic infiltrates within glomeruli were also examined and AZM198 shown a trend towards reduced glomerular capillary infiltration, as evaluated by Ly6g staining, although it did not reach statistical significance.
Figure 7 E-G from Antonelou et al, JASN 2019
Myeloperoxidase inhibition by AZM198 did not result in increased adaptive T cell-mediated immunity, which was established in an adoptive transfer model using TCR transgenic T cells. ELISA for IgG1, IgG2b, and IgG3 subclasses showed no difference with AZM198 treatment compared to vehicle controls (Figure 8, data not shown). Flow cytometry of splenic CD4+ T cells (which are primarily T follicular helper T cells), did not show an increased activation phenotype (measured by high cell surface expression of CD44 and reduced expression of CD62L). Finally, antigen-specific T cells derived from draining lymph nodes in the DO11.10 mice immunized with ovalbumin peptide did not show an increased activation phenotype (Figure 8, data not shown). These studies were performed because patients with genetic MPO deficiency have augmented antigen-specific T cell responses, although there is no evidence that this occurs with MPO inhibition with AZM198.
Take home points
The in vitro studies with human sera shown that MPO inhibition:
Reduces production of reactive oxygen intermediates
Reduces human neutrophil peptide levels
Reduces NET formation
Reduces endothelial cell damage
The in vivo studies in murine models shown that AZM198:
Reduced proteinuria and serum creatinine in a murine model of nephrotoxic nephritis
Resulted in reduced macrophage and CD4+ T cell infiltration within glomeruli
Reduced glomerular capillary loop fibrin thrombi
Reduced severity of nephrotoxic nephritis, with no anuric or deceased animals in the AZM198 group.
These data suggest that AZM198 could be a promising treatment target for ANCA-associated GN, and could potentially benefit other forms of active GN, as crescentic IgA nephropathy, proliferative lupus nephritis, and ANCA-negative crescentic glomerulonephritis also show MPO deposition.
Summary by Tiffany Caza
Nephropathologist & NSMC faculty
Arkana labs, Little Rock











![Figure_7_remaining[1].png](https://images.squarespace-cdn.com/content/v1/535bcb2fe4b05fe61b320c51/1581372152196-8SDOAPXF4DF0M71Q0E9K/Figure_7_remaining%5B1%5D.png)
