“We’ve been wrong about what our job is in medicine. We think our job is to ensure health and survival. But really it is larger than that. It is to enable well-being.”
― Atul Gawande, Being Mortal: Illness, Medicine and What Matters in the End
As nephrologists, we master and expertly apply our knowledge of homeostasis, electrolyte, acid-base and fluid balance to steer patients living with kidney disease through a long and arduous journey of CKD, dialysis and hopefully transplantation. Physiology is no doubt the pride of nephrology but sometimes it is all too easy to have a laser sharp focus of the pathophysiology and pharmacological treatment of the disease at hand and lose track of the patient’s day-to-day experience through this churn. We trend our patients’ calcium and phosphorous, hemoglobin and albumin, Kt/V and PTH month by month and adjust the medications and dialysis parameters carefully to keep these numbers “at goal”. But what about the fatigue, the pain, the cramps, the poor sleep and the sexual dysfunction? Are there any objective measurements of these symptoms? These are just a few of the many reasons why the quality of life of dialysis patients is poor. These factors are hugely important to the patient but are not generally reflected in the sheet of lab results we focus on at dialysis rounds. This is a huge discord!
A study by Urquhart-Secord et al (AJKD Sep 2016) found that while trials in hemodialysis have typically focused on outcomes such as death, adverse events, and biological markers, patients tend to prioritize outcomes that are more relevant to their daily living and well-being. This gave birth to Standardized Outcomes in Nephrology (SONG) initiative to establish core outcomes set to be consistently measured and reported in hemodialysis trials. SONG aims to improve the integrity, transparency, usability and contribution of research relevant to patients requiring hemodialysis and to ensure that outcomes of relevance to patients, caregivers, and health professionals are consistently reported across trials.
Figure from SONG HD
What are PROs, PROMs and PREMs?
A patient reported outcome (PRO) is directly reported by the patient without interpretation of the patient’s response by a clinician or anyone else and pertains to the patient’s health, quality of life, or functional status associated with health care or treatment.
Patient reported outcome measures (PROMs) are validated questionnaires developed for patients to directly report how they function and feel with respect to a health condition and related treatment, avoiding interpretation of their responses by a health care provider. These questionnaires are generally self-filled and include questions that measure functional status, health related quality of life, symptoms and symptom burden, and health-related behaviors such as anxiety and depression.
Patient-reported experience measures (PREMs) are instruments that report patient satisfaction scores with a health service and are generic tools that are used to capture the overall patient experience of health care.
PROMs: the past and the present
Some of the early references to patient reported outcome measures are in the prestigious Massachusetts Medical Society’s Shattuck Lecture of Dr. Paul Ellwood in 1988 (NEJM, June 1988). Dr. Ellwood, who is considered to be the father of the health maintenance organization (HMO) in the United States, devoted his lecture to ‘Outcomes Management’. He defined Outcomes Management as a technology to describe institutional arrangements for measuring outcomes based on patient experience and making the results available to patients and providers. Routine and systematic measurement of well being and functioning of patients at appropriate time intervals along with disease specific clinical outcomes is one of the principles of outcome management. The medical outcomes study published in JAMA in 1989 was at the forefront of this concept. In the 1970s the medical care system in the United States was being restructured, with the goal of containing rising health care expenditures but little attention was being paid to how patients' health and level of functioning in everyday activities was affected by the health care reforms. The medical outcomes study concluded that there are good reasons for developing more practical tools for monitoring patient functioning and well-being in clinical practice and in research- “Such routine assessments would be useful in detecting and explaining decreased functional capacity, keeping track of changes in function over time, making it possible to consider the patient's total functioning better in choosing among therapies, guiding the efficient use of community resources and social services, and predicting more accurately the course of chronic disease”.
Fast-forward to the present- what is considered of foremost importance today is a value based and personalized or patient-centered approach to health care which strongly beckons an efficient and sustainable way to collect and use PROMs to make a meaningful impact on patients’ health.
In the decades following the health care reforms of the 1970s, patient reported outcomes were increasingly being recognized in drug development research and the pharmaceutical industry recognized the importance of considering patient reported outcomes along with biomarkers of health improvement.
In 2004, National Institutes of Health (NIH) initiated a multicenter cooperative group referred to as Patient-Reported Outcomes Measurement Information System (PROMIS). The goal of PROMIS was to build and validate common, accessible item banks to measure key symptoms and health concepts applicable to a range of chronic conditions. It was designed to develop tools which would enable efficient and interpretable applications of patient reported outcomes in clinical practice and clinical trials. The US Food and Drug Administration (FDA) and the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) PRO extension have emphasized the need to include PROMs as trial endpoints.
What is the status of PROMs in nephrology?
A Technical Expert Panel (TEP) convened by CMS in 2013 recommended that ESKD quality metrics include dialysis-specific Health Related Quality of Life (HRQoL) and functional status to better ascertain the tolerability of treatments. Freely available PROMIS instruments which use computer-adaptive testing were viewed as feasible and sustainable PROMs.
HRQoL is now required to be routinely assessed among patients of in-center hemodialysis facilities as part of the Conditions of Coverage. The 2015 ESKD Prospective Payment System final rule identified several examples of PROMs to help assess patients for major depressive disorder. Screenings for pain and depression were included into the 2018 End-Stage Renal Disease Quality Incentive Program.
Recognizing that PROMs are an increasingly key component of patient-centered kidney disease care, Devika Nair and Perry Wilson (AJKD Sep 2019) did a thorough review of a list of PROMs developed for adults with kidney disease with limitations of each measure, ongoing initiatives and prior work related to the incorporation of PROMs into nephrology clinical trials as well as health care policy and challenges to seamless incorporation and uptake of PROMs in nephrology. They noted that the majority of clinical trials in nephrology that have PROMs as endpoints do not include them as primary endpoints, and only a few of them originate in the United States.
In 2018, in an effort to standardize PROMs in nephrology, International Consortium for Health Outcomes Measurement (ICHOM) assembled an international working group of health professionals and patient representatives to develop a standardized outcome set in CKD for integration into routine clinical practice including PROMs (AJKD, March 2019). This set consists of 12 outcome domains, 4 of which are PROMs in the “essential Tier 1” and were regarded as most important by patients.
Figure from Verberne et al, AJKD March 2019
Nephmadness 2018 inevitably crowned PROMs as the champion that year!
Okay, so we recognize PROMs are important, and have ways to measure them, but does routine collection of PROMs improve kidney patients’ outcomes?
What evidence do we have to suggest this and if so, how do we implement this in our routine clinical practice?
By letting the patients be in charge of reporting their symptoms and outcomes of treatments, PROMs can allow patient-centered approach to nephrology care and may improve our ability to design individualized treatments.
But there are certain prerequisites to unlock the promise of PROMs:
PROMs need to be collected consistently over a long period of time.
There needs to be infrastructure in place to efficiently collect, store, process and report PROMs data.
As one can imagine, this is a difficult job!
So while the use of PROMs is being encouraged in nephrology, effective implementation of PROMs collection to improve routine clinical practice is still challenging and in its nascent stages. A lot of creativity and innovative thinking on part of PROMs researchers has gone towards trying to develop efficient ways of using PROMs within an existing clinical trial’s framework.
This is exactly what is so unique about the Symptom Monitoring With Feedback Trial (SWIFT) as well as other pragmatic trials currently in progress attempting to strengthen the evidence of benefits of routine PROMs collection in CKD.
In what has been called trailblazing work, two pilot studies in Sweden (Pagels et al 2019) and the Netherlands (van der Willik et al 2020) first demonstrated that national kidney registries can be used to collect PROMs during routine dialysis care. An accompanying editorial by van der Veer et al (CKJ 2021) has beautifully shed light on how kidney registries can be effectively used for PROMs collection and feed back results to improve care of CKD and dialysis patients.
How are national kidney registries an excellent resource for PROMs collection ?
National kidney registries are databases that systematically collect, store, analyze and report information about kidney disease in a standardized way. We covered this in our previous #Nephtrials on registry based clinical trials.
The pilot studies conducted in Sweden and the Netherlands used their respective national kidney registries which provided the infrastructure to capture and report HRQoL data in people with chronic kidney disease. The kidney registries have a capability to longitudinally track patient outcomes and therefore can be used to collect, process and report PROMs.
Van der Veer and others have highlighted several important ways in which kidney registries can help PROMs collection:
Registry based PROMs collection can allow a standardization of PROMs instruments use, mode and frequency of collection across different dialysis centers within a registry.
It could also facilitate linkage of PROMs data to clinical and administrative data sources
It can allow integration of PROMs as an outcome in registry-based trials
Feasibility studies such as the SWIFT pilot study have further enhanced our understanding of how kidney registries and healthcare providers could collect and use PROMs successfully as part of routine care. The SWIFT pilot study, completed in 2020, was done in 4 dialysis units in South Australia and Queensland and concluded that electronic symptoms monitoring in patients on hemodialysis using tablet computers, with feedback to clinicians, was feasible.
The Dutch and Swedish pilot studies reported a low average PROMs response rate, with substantial between center variation and suboptimal patient and staff engagement. These have been identified as key barriers to successfully introducing PROMs in routine kidney care. These pilot studies in Europe and Australia have successfully brought to the forefront the robust infrastructure that national kidney registries can provide to enable PROMs collection and use in routine practice.
Figure from van der Veer et al (CKJ 2021)
The SWIFT trial
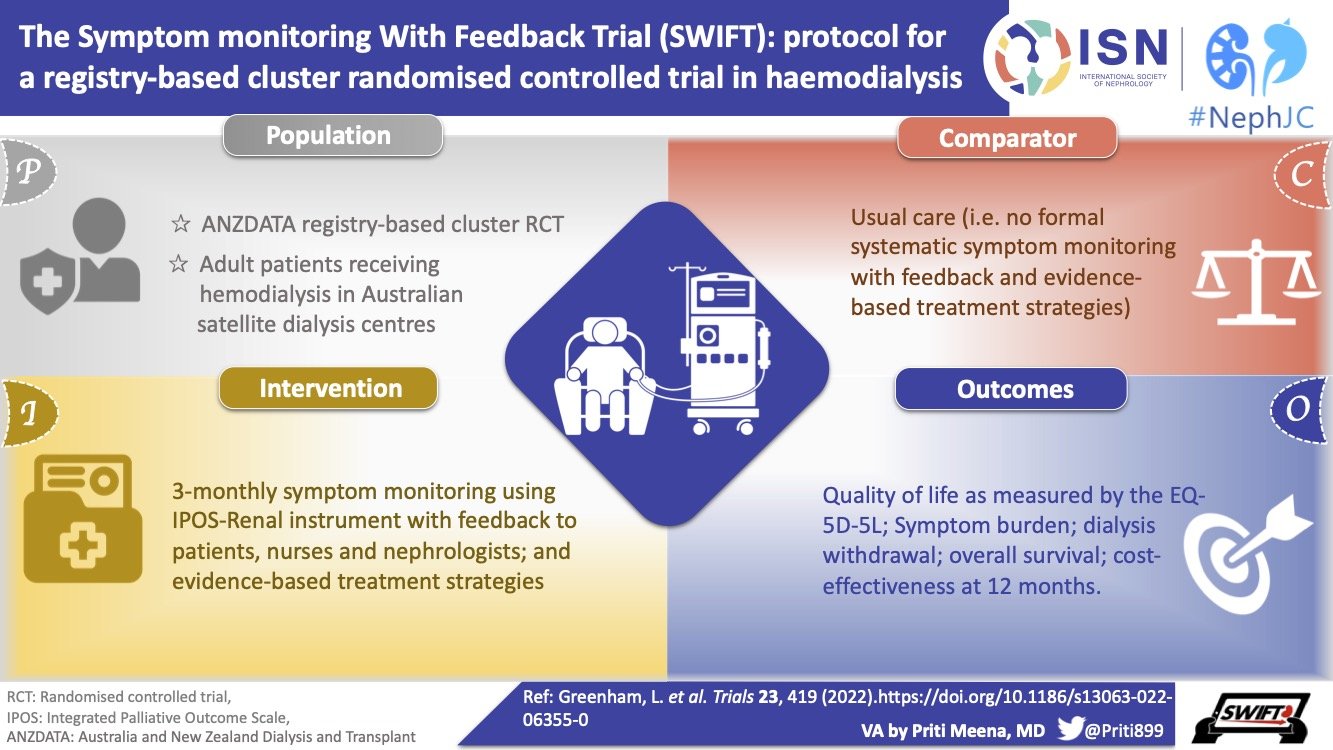
A Visual Abstract of the SWIFT Trial methods, by Priti Meena, ISN Social Media team
SWIFT is a registry-based pragmatic cluster RCT. This trial is designed to assess the clinical and cost-effectiveness of regular symptom monitoring in patients receiving in-center hemodialysis, with feedback to clinicians which is embedded within ANZDATA (Australia and New Zealand Dialysis and Transplant Registry). The objective is to evaluate the hypothesis that regular symptom monitoring with feedback to patients and clinicians improves QOL at 12 months.
As is generally with pragmatic cluster trials, the eligibility criteria are quite broad and inclusive. Patients undergoing hemodialysis in all centers participating in the ANZDATA Registry who were willing to adhere to the trial requirements and were willing to give informed consent have been invited to participate.
The trial plans to recruit 143 hemodialysis centers in Australia, with approximately 71 dialysis centers in each arm, equating to 2422 total participants. Regardless of the dialysis provider, all dialysis care is free to the patient and all dialysis units send patient data to the ANZDATA registry.
Usual care in the dialysis unit is the control arm in comparison to the intervention arm in which patients will be administered the Integrated Palliative Outcome Scale (IPOS-Renal) survey at baseline and at every 3 months.
Both arms will collect health-related quality of life through the EQ-5D-5L instrument as well as the SONG-HD fatigue measure at baseline, 6 months and 12 months. All PROMs will be collected during routine hemodialysis sessions electronically on an Android tablet provided to the dialysis unit. The materials have been translated in 7 languages other than English.
Figure from Greenham et al Trials, May 2022
Successful completion of SWIFT will facilitate ANZDATA registry to expand on the outcomes collected to include PROMs if the trial determines this to be beneficial. This will complement dialysis services that currently have infrastructure of national or international registries for collecting and reporting information on all patients receiving treatment.
The primary outcome of SWIFT is a change in health-related QOL as measured by the mean change in EQ-5D-5L value from baseline to 12 months. Other secondary outcomes are listed in the table below.
Figure from Greenham et al Trials, May 2022
What's in the future of PROMs in nephrology?
There are other ongoing pragmatic trials on PROMs collection in CKD-
EMPATHY: To determine the effects of routinely measuring PROMs on the experiences of patients undergoing hemodialysis in Alberta and Ontario.
PROKID: The aim of this study is to compare the effect on the clinical outcomes, the utilization of resources, and patient-reported outcome in three types of follow-up in a non-inferiority randomized controlled trial.
The previously completed Dutch and Swedish pilot studies and ongoing Australian SWIFT study are set to pave the way for other kidney registries to harness the registry infrastructure and support pragmatic comparative effectiveness studies to evaluate the benefits of PROMs based interventions. Van der Veer et al in their editorial anticipate that the fast-growing evidence base on whether, and how, PROMs can be of value in CKD settings will expedite the uptake of routine PROMs collection by other kidney registries and healthcare providers across the globe.
This 2018 Neph Madness commentary on PROMs by Dr. Finkelstein pretty much sums it up.
One of the quotes from that year’s Blue Ribbon Panel that stuck with me most was this,
“Surrounding every kidney is a living person”
Hopefully, the uptake of PROMs in innovative clinical trials such as SWIFT and others will ultimately lead to more valuable and patient-centered CKD care and enhance the health and well-being of our patients.