#NephJC Chat
Tuesday June 26 9 pm Eastern
Wednesday June 27 8 pm BST, 12 noon Pacific
Lancet. 2017 Nov 11;390(10108):2160-2170. doi: 10.1016/S0140-6736(17)32281-X. Epub 2017 Aug 28.
Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial.
Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M; SPYRAL HTN-OFF MED trial investigators*.
PMID: 28859944
Introduction
In November 2017, SPYRAL HTN-OFF became the first of the three new renal denervation trials to be published. The methods and the rationale (for this as well as SPYRAL HTN-ON) are presented in this methods paper. As discussed in the introductory historical background post, one of the recommendations after reviewing the variance in blood pressure studies which increased with increasing medications, was to do a trial in patients who were not on any anti-hypertensive medications (hence 'OFF'). This trial served as a proof-of-concept to demonstrate that, in the background of no blood pressure medications, renal denervation did indeed lower blood pressure. To test if renal denervation actually did lower blood pressue at all - and that it was not just regression to mean and other artifacts at play. All key aspects from Symplicity HTN-3 were also incorporated, mainly the use of sham controls and the use of ambulatory blood pressure monitoring.
The Study
Methods
This was a multicentre study, done in 21 centres over th globe: US, Europe, Australia and Japan.
Inclusion Criteria
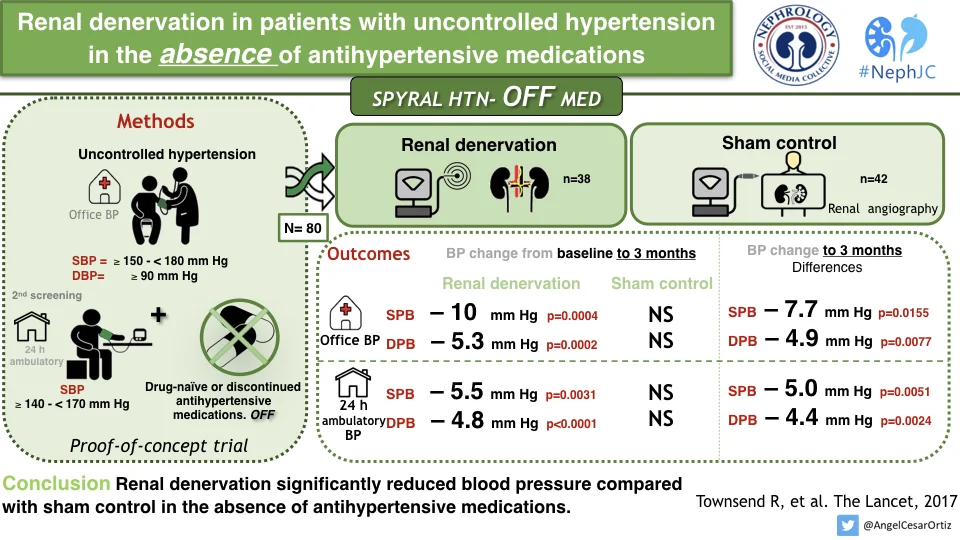
The patients were 20-80 years of age with mild to moderate hypertension. Moderate hypertension was defined as:
Office systolic blood pressure (SBP) ≥150mmHg and <180 mm Hg
Office diastolic blood pressure [DBP] ≥90 mm Hg
A mean 24-hour ambulatory SBP ≥140 mm Hg and <170 mm Hg
They needed to either be on no blood pressure lowering medications - or if they were on, required a 3-4 week wash-out period. The latter aspect was, interestingly, tested by urine and plasma testing at baseline and 3 months to ensure no one was surreptitiously or mistakenly ingesting an antihypertensive medication. After the washout, a second screening visit confirmed that patients were still eligible for randomisation.
Randomisation to renal denervation or sham procedure was stratified by trial centre with a 1:1 ratio.
The Procedure
After randomization the patient underwent a renal angiogram (sham procedure) or renal angiogram with a subsequent radiofrequency ablation renal denervation procedure. Like in Symplicity HTN-3, patients were blinded during the renal angiogram by a combination of conscious sedation, sensory isolation (blindfolding and music), and absence of familiarity to the procedural details and duration of the angiogram.
Multi-electrode catheters were used (unlike the original Symplicity catheter) for circumferential and longitudinal ablation of the renal nerves. Also, all branch and accessory arteries (with diameter between 3 and 8 mm) were targeted for ablation.
Figure 1 from Townsend et al, Lancet 2017
Measurements and Analysis
The patient’s blood pressure was assessed every 2 weeks after randomisation. Since they were not on treatment, there were prespecified escape criterion (SBP >/= 180 mm Hg, confirmed by repeat measurement < 72 hours) that allowed initiation of antihypertensive medications.
The primary efficacy outcome was the change in BP from baseline, comparing RDN versus sham groups. For baseline, BP from the second screening visit was used. Given that this was a proof-of-concept to demonstrate that renal denervation actually did something, there was no formal sample size calculation, nor a prespecified effect size. Instead, analyses were prespecified at 40, 60, 80, or 100 patients followed up for 3 months so that if a clinically meaningful reduction was observed there could be rapid advancement to the design and initiation of a larger, powered, pivotal study. This report includes data on the first 80 patients with 3 month of follow-up.
Funding
The trial was funded by the maker of the Symplicity and Spyral catheters: Medtronic. They were responsible for data collection and analysis as well as assisted in figure and table generation, copy editing, and formatting.
Results
Between June 25, 2015, and Jan 30, 2017, 353 patients were screened and enrolled. This interim analysis presents results for the first 80 patients randomly assigned to renal denervation (n=38) and sham control (n=42). A few patients did reach escape to need BP medications, as below.
Figure 2 from Townsend et al, Lancet 2017
Overall the patient population was low risk, with mean age of 50 years, little diabetes or other comorbid conditions. The office BP at baseline was about 160/100 mm Hg and the 24-hour ABPM were 152/100 mm Hg.
To belabor the point of more extensive ablations (compared to Symplicity HTN-3), an average of 43.8 ablations were done per patient, of 2.2 main arteries and 5.2 branch vessels! The blinding was effective, with an index of 0.65 at discharge and 0.59 at 3 months (> 0.5 is evidence of effective blinding).
The 3 month change in SBP and DBP for both the ambulatory BP and office BP measurements was signifigant.
figure 3 from Townsend et al, Lancet 2017
An interesting way to see individual patient responses - beyond the overall average response, is shown in the figure below. As can be seen, not every participant had a decrease in BP with renal denervation - despite the extensive ablations mentioned above.
Figure 4 from Townsend et al, Lancet 2017
Safety: From this standpoint, there were no events - procedural or clinical - though the period of follow up was somewhat short.
Discussion
The authors conclude that this trial showed a clinically significant reduction in office and 24 hours ambulatory SBP and DBP at 3 months in patients with mild to moderate renal hypertension in the absence of anti-hypertensive drugs, an effect which was not seen in the sham control group. These results provide proof-of-principle for the efficacy of catheter based renal denervation to reduce the BP in patients not on anti-hypertensive drugs.
Strengths of the study include
Use of sham procedures and effective blinding
Use of rigorous BP measurement (ABPM at baseline and follow up)
Significant effort made to ensure adequate nerve ablation based on procedural details
Limitations include
Small sample size, with no a priori assumption of effect size (deliberate)
Low risk population (again, planned and deliberate)
Hence, this trial demonstrates that the denervation strategy used results in a meaningful decline in blood pressure at 3 months in a low risk, but hypertensive population with no safety signal.
Check out our overall summary for comparisons with the other studies and discussion plan.
Summary by Mayuri Trivedi, Nephrologist, Mumbai
NSMC Intern Class of 2018
and Swapnil Hiremath, Nephrologist, Ottawa, Canada