#NephJC Chat
Tuesday June 26 9 pm Eastern
Wednesday June 27 8 pm BST, 12 noon Pacific
Lancet. 2018 Jun 9;391(10137):2335-2345. doi: 10.1016/S0140-6736(18)31082-1. Epub 2018 May 23.
Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L; RADIANCE-HTN Investigators.
PMID: 29803590
Introduction
The RADIANCE SOLO trial is part of a group of studies looking at the effect of a different (compared to catheter ablation in the SYMPLICITY and SPYRAL system from Medtronic) method of ablation of the sympathetic nerves around the renal artery. This technique uses ultrasound to perform thermo-ablation, and was developed by ReCOR Medical, using what they term the 'Paradise' system. See the video below to see how this works.
Like the SPYRAL HTN-OFF system, the RADIANCE HTN SOLO trial was designed to be in patients with mild hypertension, who would be off treatment. Also, like Symplicity-3 and the pair of SPYRAL trials, they were blinded, randomized, controlled trials (RCT) with sham procedures and with ambulatory blood pressure monitoring (ABPM) based outcomes.
The Study
Methods
This was a multicentre trial, involving 39 centres from US and Europe (including UK).
Study Population
Age 18 to 75 years
Office BP ≥ 140/90 but < 180/100
on zeron, one or two BP medications
GFR > 40 ml/min
If patients were on medications, they were required to stop them for 4 weeks before undergoing the qualifying ABPM study. This needed to be ≥ 135/85 and < 170/105 to be randomized into the study. Unlike SPYRAL, no assessment of adherence (eg drug level from blood/urine) were conducted. All patients remained off antihypertensive medications until 2 months after randomisation unless office blood pressure reached 180/110 mm Hg or home blood pressure reached 170/105 mm Hg before the 2-month evaluation, in which case patients received escape antihypertensive treatment.
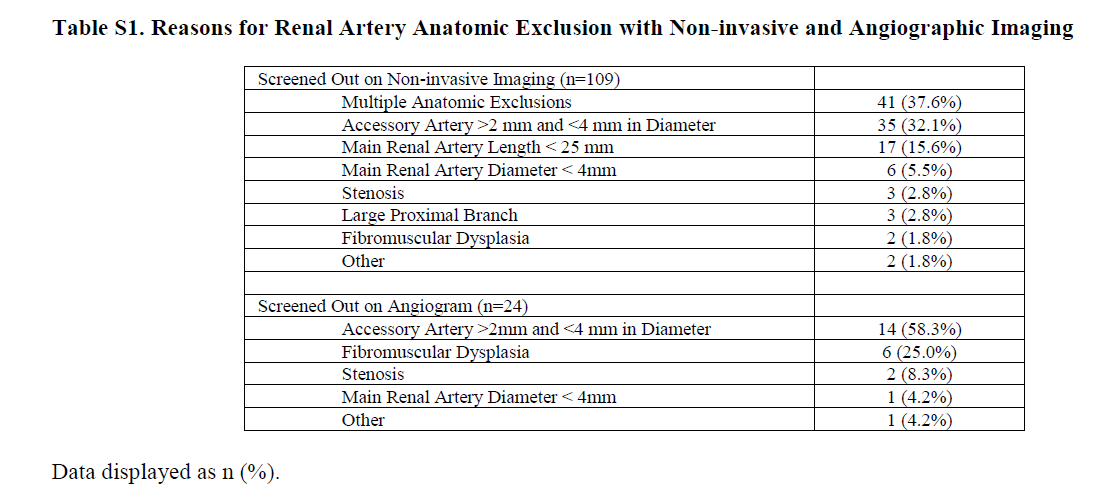
Suitable renal anatomy was the other eligibility criteria. Given the nature of the intervention, renal artery size and length had to be sufficient, and patients with large branches or accessory arteries were excluded:
Table S1 from Azizi et al, Lancet 2018
Outcome and Analysis
The primary efficacy outcome was change in 24-hour ABPM between groups at 2 months, compared to baseline, in between groups, as per intention to treat principle. The ABPM were sent to a different blinded centre for analysis - but it is not mentioned if the research personnel measuring office BP and performing the ABPM were blinded to treatment assignment. Patients were provided a home BP monitor and encouraged to measure home BP for 7 consecutive days before every visit.
Unlike SPYRAL, there was an a priori sample size estimation: Assuming a 6 mm Hg difference in change in daytime ambulatory systolic blood pressure at 2 months between the renal denervation and the sham groups, a common SD of 12 mm Hg, 1:1 randomisation, and a two-sided type 1 error rate of 5%, a sample size of 128 evaluable patients would yield 80% power. To account for up to 10% missing data on the primary endpoint, they planned to randomise a total 146 patients in the study.
Funding Source
The study was funded by ReCor Medical, the device manufacturer. They were responsible for data collection as well as assisted in figure and table generation, copy editing, and formatting.
Results
Overall, 803 patients were screened to randomize about 146 patients into the trial. Figure 1 below shows the flow - note that many patients did not fulfil renal anatomy criteria, 109 in first step and 24 in the second, for various reasons (does not make study invalid: only one cannot generalize to those without suitable anatomy). One patient in the intervention group and three patients in the sham procedure reached escape criteria (elevated BP) and required antihypertensive medications. Four and ten patients, respectively, were treated on the basis of the physician's decision or patient preference, despite not meeting protocol-defined criteria.
Figure 1: Study flow from Azizi et al, Lancet 2018
From table 1, the baseline characteristics are similar to the two SPYRAL trials: mean age around 50, and few comorbidities. About 80% were on 1 or 2 medications before washout. At baseline office BP was about 154/100 and 24-hour ABPM was about 150/93. So what happened to blood pressures after intervention? See main results (intention to treat) at 2 months below.
Figure 2 from Azizi et al, Lancet 2018
The decline in 24-hour ABPM between groups was about 4/2 mm Hg. There was also a decline in BP in the sham group.
Given that some patients received BP lowering medications (off protocol) see the per-protocol results also below. Note that the per-protocol population excluded patients who
did not meet baseline daytime ambulatory systolic or diastolic blood pressure or renal artery anatomical inclusion criteria,
patients in the renal denervation group who did not receive bilateral renal denervation,
patients who were treated with antihypertensive medications before the 2-month ambulatory blood pressure measurement (according to protocol criteria or according to physician's or patient's decision), and
patients who did not complete the 2-month ambulatory blood pressure assessment.
The actual numbers per-protocol were a bit higher than the ITT ( -6/3 vs - 4/2 mm Hg in 24 hour ABPM between groups).
Table 3 from Azizi et al, Lancet 2018
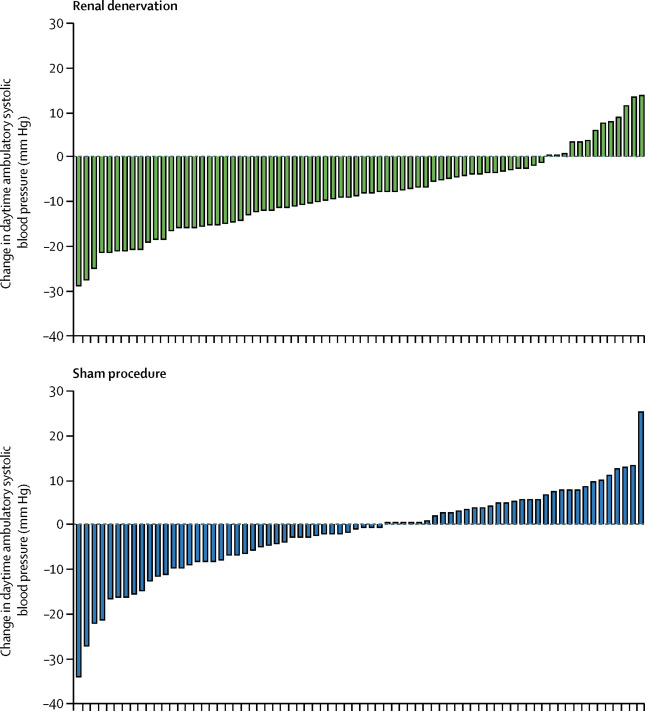
Figure 3: Individual patient response from Azizi et al, Lancet 2018
As in the SPYRAL trials, individual response varied as shown above in figure 3.
There was some exploration of the subgroups shown below. Though interaction p values are not presented, the 95% CI do overlap, so unlikely for any difference in the few compared here.
Figure S3 from Azizi et al, Lancet 2018
Was blinding effective? It was - presented in the supplementary data (James blinding index ~ 0.7 at discharge and 0.6 at 2 months).
Safety: At 2 months, there were no safety signals - and renal artery imaging did not reveal any significant (> 50%) narrowing. Interestingly, one patient in the denervation arm requires a stent at 6 months. An independent review of their preprocedural and 6-month postprocedure renal artery imaging showed that there was a pre-existing ostial renal artery stenosis (40–50% on MR angiography, 44% on renal angiography), which would have met the criteria for exclusion but was not recognised at the time of randomisation, and a 57% ostial renal artery stenosis on renal angiography before renal artery stenting at 6 months.
Discussion
Overall, as with the SPYRAL trials, the RADIANCE SOLO trial shows, that in patients with hypertension, once off antihypertensive medications, that renal denervation with the PARADISE system significantly decreased blood pressure compared to sham controls, with no safety signal at 2 months.
Strengths of the study include
Use of sham procedures and effective blinding
Use of rigorous BP measurement (ABPM at baseline and follow up)
Limitations include
Low risk population (again, planned and deliberate)
short follow up (2 months)
no effort made to ensure adherence to being off medications
Check out our overall summary for comparisons with the other studies and discussion plan.
Summary by Mayuri Trivedi, Nephrologist, Mumbai
NSMC Intern Class of 2018
and Swapnil Hiremath, Nephrologist, Ottawa, Canada