#NephJC Chat
Tuesday March 14th, 2023 at 9 pm Eastern
Wednesday March 15th, 2022, at 9 pm Indian Standard Time and 3:30 pm GMT
JAMA Intern Med. 2023 Feb 1;183(2):134-141. doi: 10.1001/jamainternmed.2022.6069. Erratum in: JAMA Intern Med. 2023 Feb 20.
Association of Pretransplant Coronary Heart Disease Testing With Early Kidney Transplant Outcomes
Cheng XS, Liu S, Han J, Stedman MR, Baiocchi M, Tan JC, Chertow GM, Fearon WF.
PMID: 36595271
Introduction
Kidneys are scarce resources, especially in the setting of a long list of patients on dialysis waiting for a precious transplant. As a way of maximizing the utility and minimizing potential waste, it is generally accepted that patients are required to be “healthy” prior to organ transplantation. This would include being free of cancers and infections, as well as many diseases that might increase peri-transplant mortality. For decades transplant centers have required cardiac ischemia evaluations as part of a comprehensive pre-transplant work-up. This adds to the long journey towards getting waitlisted (see NephMadness scouting report for more). Why do we stress a kidney transplant candidate, and ourselves, by putting them through cardiac testing? Is there substantial evidence that cardiac testing significantly reduces adverse patient outcomes in kidney transplantation? In the hunt for optimal transplant outcomes and reduced peri-transplant mortality, nephrologists have heard arguments on both sides of the aisle for the past decade. Balancing good post-transplant outcomes versus shortened evaluations and wait times is difficult.
In 2012 the AHA looked at the incidence of CHD (Coronary Heart Disease) in patients with end-stage kidney disease (ESKD), including transplant candidates, and opined that there was poor correlation between angiographic CHD and clinical outcomes. In several studies from 2003-2008 involving patients with ESKD and/or patients being evaluated for kidney transplant, CHD was present in at least 40% of individuals. However, the outcomes ranged only from no significant increase in major adverse cardiovascular events to some increase which was not statistically significant. The AHA also evaluated noninvasive cardiac testing for CHD in kidney transplant candidates; all had either poor sensitivity or specificity in the transplant population.
Table 5 from AHA Statement (Lentine et al, JACC 2012)
On the basis of these observational data, and expert opinion, several organizations (see summarized in graphic below) recommend coronary heart disease screening prior to kidney transplantation based on the underlying risk, with some variations across different countries. They also differ in their definition of low versus high cardiac risk.
The Ischemia-CKD trial (see NephJC summary) reported that patients with chronic kidney disease (CKD) did not undergo angiography as often as patients without CKD, but invasive strategies in treatment did NOT improve angina or event rates - not even in the subgroup of those listed for a kidney transplant (Herzog et al, JACC 2021). A subsequent meta-analysis (Bangalore et al, Circulation 2020) further corroborated the same findings. Hence the argument has been made that cardiac screening - especially in asymptomatic and low risk patients - merely delays and denies a kidney transplant, which is the most effective modality for these patients (Sharif, AJKD 2020 and Sharif, Kidney 360 2022). Despite the results of the ISCHEMIA-CKD trial, and these persuasive viewpoints, data on trends in the USA show that testing rates have not decreased in low risk candidates, though variation across centers exists (Cheng et al, Kidney360 2021).
In this study, we will see the impact of cardiac testing using the existing variation and a unique analytical design.
The Study
The authors of this trial conducted a retrospective observational study in a US cohort using data from the US Renal Data System. They use instrumental variable analysis to analyze whether pre-transplant cardiac testing for coronary heart disease (CHD) was associated with adverse outcomes early after transplant. They specifically looked at the primary outcome of death or acute myocardial infarction (MI) within 30 days after kidney transplant.
Methods
Study design: Retrospective registry-based cohort study with instrumental variable (IV) analysis. IV analysis is a statistical tool to draw causal inferences from observational data. The instrumental variable is an outcome associated with an outcome only through the mediation of the actual exposure of interest.
Study participants: 2 cohorts - the study cohort and IV cohort.
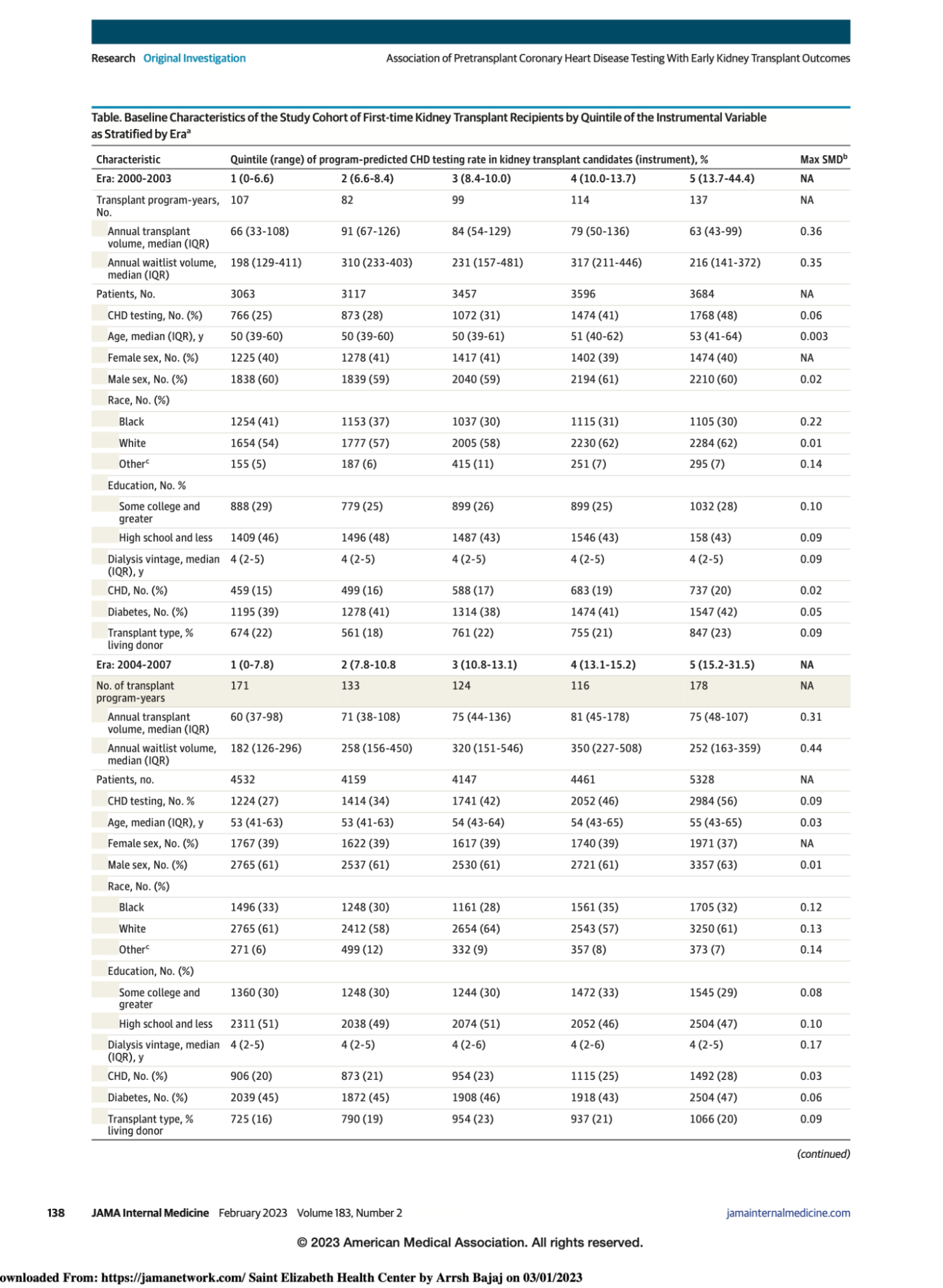
Study cohort - all adult, first-time kidney transplant recipients from 2000 through 2014 in the US Renal Data System with at least 1 year of uninterrupted Medicare Parts A and B before and after transplant (2 years total).
IV cohort - Adult, low risk kidney transplant candidates on each transplant program’s waitlist on January 1 each year.
Definition of low risk : absence of major risk factors including age >60 years, diabetes mellitus, and known CHD
Exposure: Primary exposure was non-urgent, invasive or non-invasive CHD testing, in the 12 months before kidney transplantation.
Instrumental Variable (IV) analysis: Instrument is the ‘transplant center’. IV ranged from 0 (no low risk candidate tested) to 1 (every low risk candidate tested).
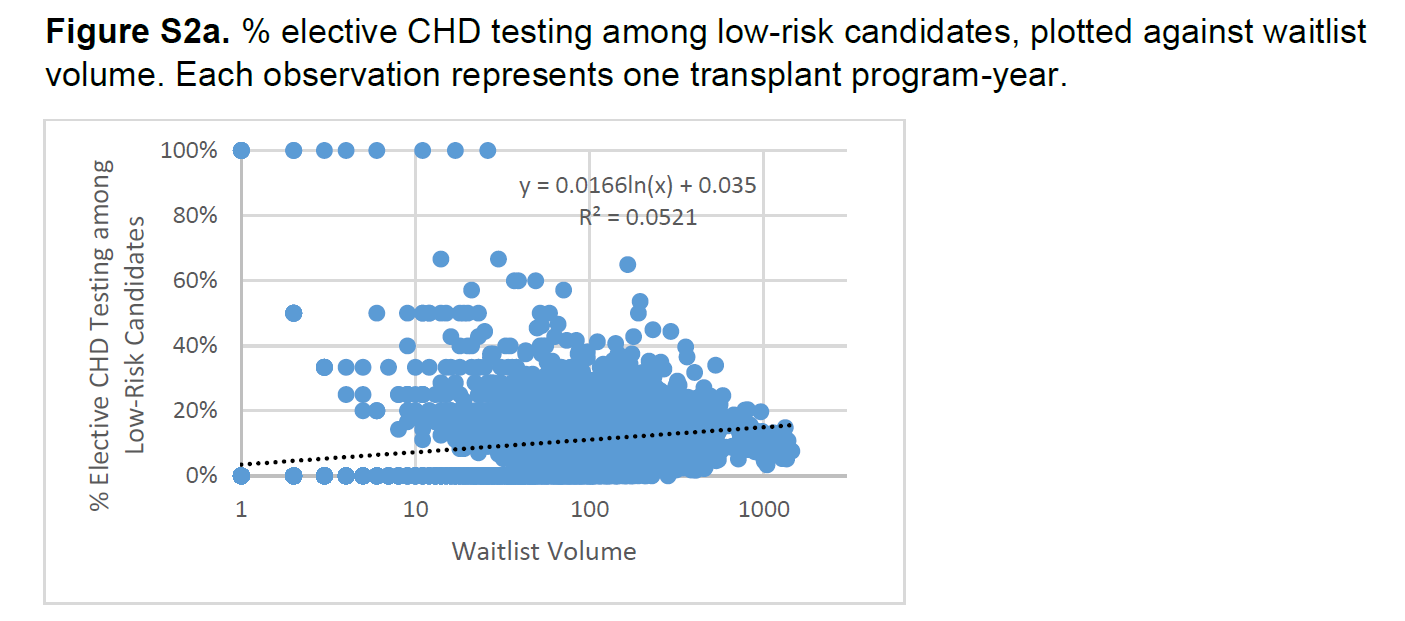
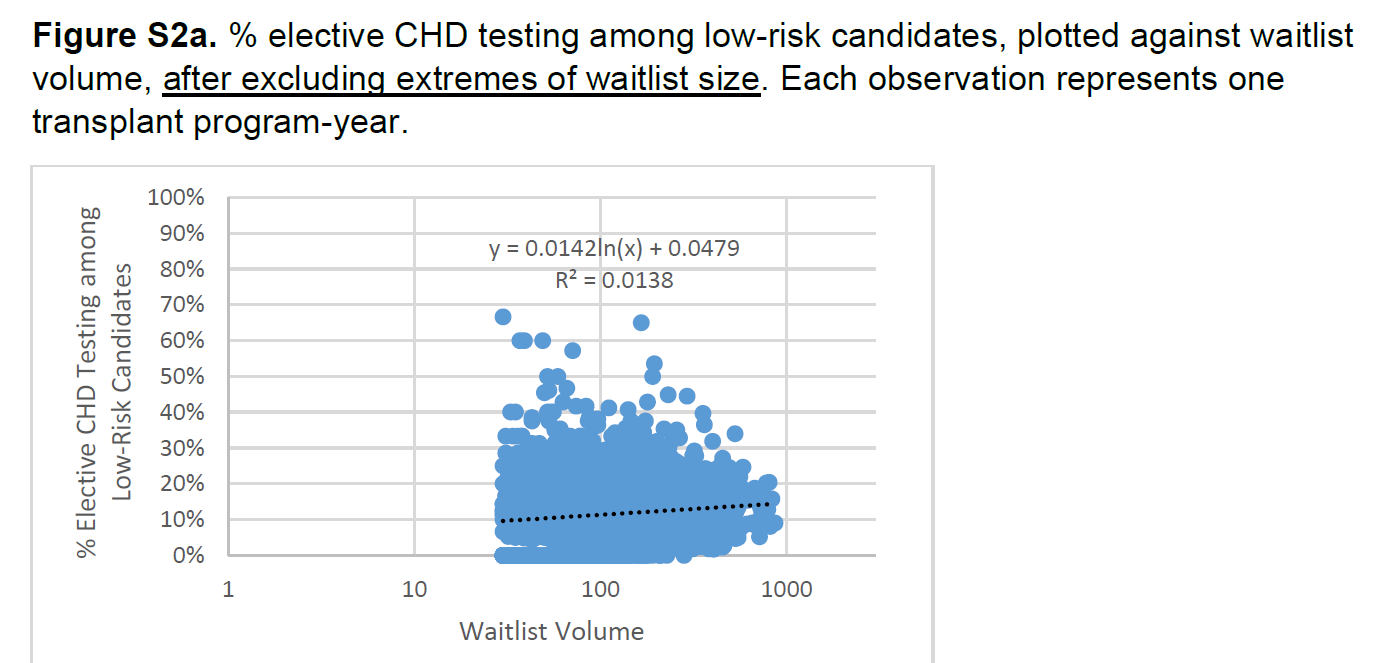
Exclusion: Since IV should detect meaningful differences in outcomes, clusters of program-years with good spread of IV were needed. Transplant volume was a potential confounder and hence program size of <30 (25th percentile) or >868 (99th percentile) were excluded (explained in more detail in the supplement, see figures below).
Outcomes: Composite of death or acute MI within 30 days of kidney transplant adjusted for age, sex, race, education, dialysis vintage, history of CHD, diabetes, and transplant type (living versus deceased donor). Outcomes were analyzed based on covariates across quintiles.
Funding: The study was funded by grants from the AHA and the NIDDK, who had no active role in the study itself.
Results
The authors examined outcomes in 79,934 kidney transplant recipients who were linked to the IV cohort as described in methods.
Figure 1 from Cheng et al, JAMA IM 2023
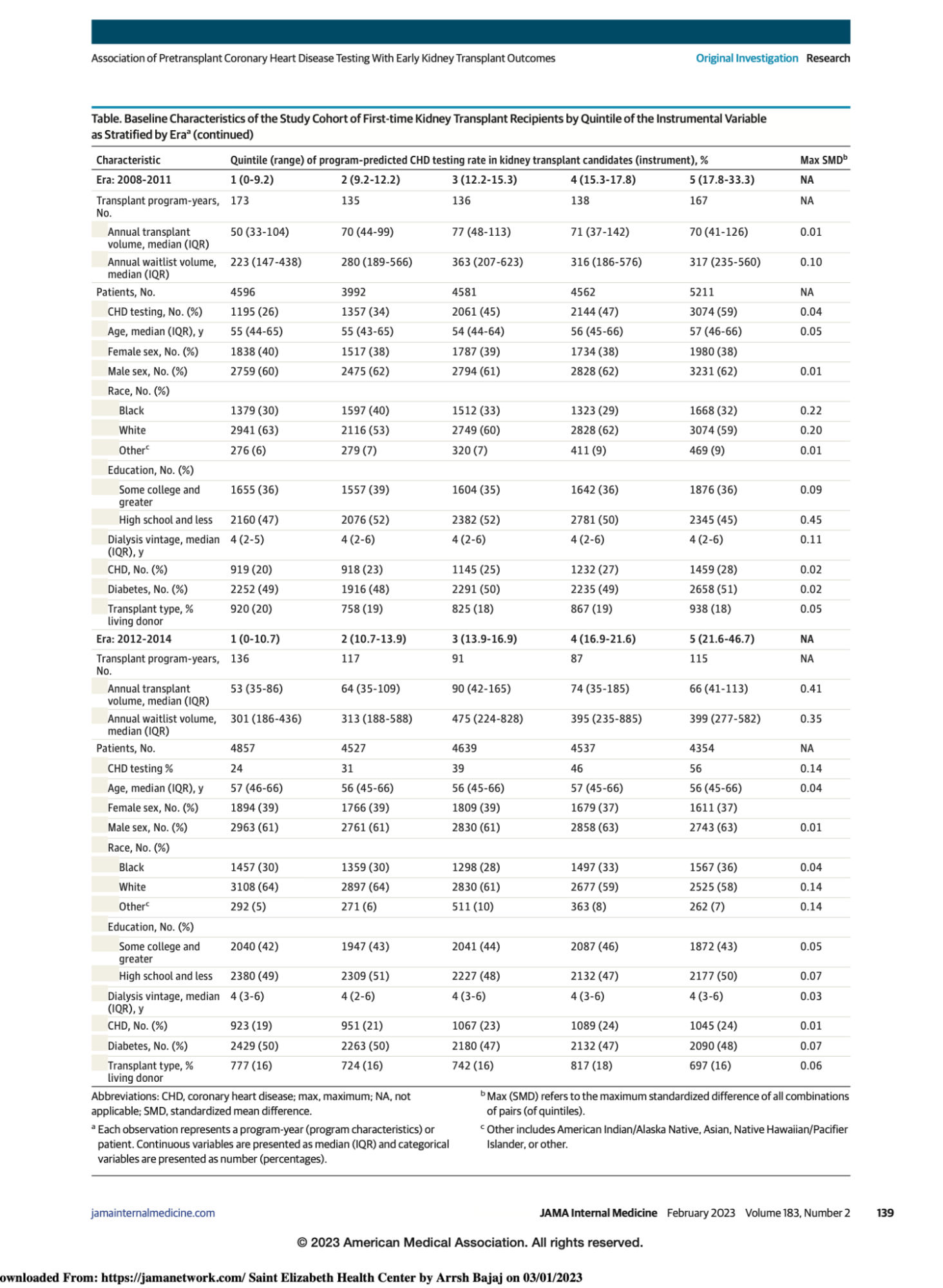
They divided the cohort into four eras based on 4 year intervals, and the data is presented based on quintiles of center level CHD testing rates (ranging from 0 to ~ 47%).
Table 1 from Cheng et al, JAMA IM 2023
As can be seen, across the quintiles, there are some differences in the populations which vary over the different eras, but the standardized mean differences (SMD, last column) was <0.2 for most variables.
CHD testing and trends over time
As we go across eras, we notice certain trends. Overall CHD testing rate is at least 25% and increasing along quintiles. There is also an increase in transplant waitlist volume across eras for the same median dialysis vintage of 4 years. At least 40% of patients in all eras had diabetes. The authors noted that 8125 patients (23% of those tested) had CHD testing on or before joining the waitlist, whereas 26,563 (77% of those tested) had CHD testing after joining the waitlist.
Outcomes
4604 patients (5.3% as stated in the paper, but 4604/79934 = 5.8%) in the study cohort reached the primary outcome (2063 patients or 2.6% for death, and 2329 patients or 2.9% for acute MI - which actually adds up to 4392, not 4604) within 30 days after a kidney transplant. The 30 day study event rate decreased from 6.6% in the first era to 4.4% in the last era. However, when one compares this to the reference of no pre-transplant CHD testing, it was not associated with a change in the rate of primary outcomes, as seen in Figure 2 (except for era 2000-2003). In the first era, paradoxically, testing for CHD was associated with a slightly higher post-transplant event rate.
Figure 2 from Cheng et al, JAMA IM 2023
Supplemental eAppendix 4, shown below, further validates this. The confidence intervals with covariates include zero, therefore rejecting the hypothesis that pre-transplant CHD testing is associated with a decrease in the primary outcome, again except for the first era of 2000 to 2003, in which the slightly higher event rate was observed. A sensitivity analysis using the converse definition of IV (program-level testing in high-risk candidates) also showed similar rates (eAppendix 5).
eAppendix 4 from Cheng et al, JAMA IM 2023
Discussion
The commonest cause of mortality in CKD is cardiovascular disease . Moreover, many patients with CKD remain asymptomatic with a high prevalence of silent myocardial ischemia, owing to factors such as diabetes and uremic neuropathy. This is the proverbial fig leaf that leads to high rates of pre-transplant CHD testing despite the lack of strong data and guideline recommendations to only test in high risk patients.
Using Medicare claims data and instrumental variable (IV) analysis, the authors report that pre-transplant CHD testing was not associated with significant reduction in death or acute MI within 30 days of transplant. In fact, they found a non-significant increase in adverse events at centers with higher rates of CHD testing.
The perioperative event rate was 5% in this study, in comparison to 3% described in the ACC-AHA guidelines for non-cardiac surgery (Smilowitz et al Cardiol Rev 2019). Two points merit discussion here. Firstly, this seemingly high event rate may indeed bias the study towards CHD screening. Secondly, the event rate in those who were screened and subsequently did not undergo transplantation is not available, and hence goes unreported. These are the counterfactuals - patients who due to screening may have been denied a kidney transplant from which they would have gained net benefit - or conversely, patients who had they been transplanted may have had a post-transplant CV event - and the CHD testing helped dodge a bullet.
It is true that unnecessary testing especially in the asymptomatic individual may lead to more invasive testing and uncalled for interventions which may be counter-productive in several ways. In fact, in this study screening was associated with an increased event rate in the early 2000-2003 era, when the standard of care in PCI was bare metal stents. It is possible that this reflects the in-stent thrombosis and higher adverse CV outcomes associated with bare metal stents (Bangalore S et al Cath Cardiovasc Inter 2015; Changal et al, Cardiovasc Revasc Med 2021). Waiting for screening and stenting also introduces inherent logistical problems with getting them done first, and in some cases may even result in subsequent delisting, or death on the waitlist.
The ISCHEMIA-CKD trial also did not provide any data to support invasive as opposed to conservative strategy for managing CKD patients - however it was not designed to address the specific question of testing in asymptomatic and low risk patients prior to transplant listing. The ongoing CARSK trial (Ying et al, Am Heart J 2019) is not aiming to address this question either - rather the question of additional surveillance testing in already waitlisted patients. It seems - until now - there has been no equipoise to randomize patients to testing versus no testing prior to transplant waitlisting. Perhaps this study will move the needle a bit more to making such a trial palatable?
This study lends additional, albeit non-RCT, evidence that asymptomatic patients may go on to have transplants without getting screened, an area as yet unexplored by RCTs. In fact, cardiac screening pre-transplantation all along has been based on good intention more than good evidence.
Strengths
Construction of IV and its application to large registry/mediclaim based data, as an effective tool that partly substitutes for an RCT.
High event rate (5%) in this study provides ground that would be favorable for showing benefit from cardiac testing, but in spite of this results showed that cardiac testing was not beneficial.
Limitations
Residual confounding is not completely resolved.
Transplant volume differs in transplant programs with different rates of CHD testing, and volume is usually inversely associated with outcomes (called collider bias - see figure below source from catalogue of bias, and also Tonnies et al 2022 ). In this study, however, the association between transplant volume and CHD testing rates is not monotonic: programs with intermediate levels of CHD testing have the highest volume. Because the trends of transplant volume and CHD testing are not consistent with each other, it is unclear if collider bias can fully invalidate this IV analysis study.
Most patients are deceased donor transplant recipients (84%). Living donor transplant may be a different ballgame altogether. There is more time and generally better planning, so this study’s overall result may not be representative of programs that rely heavily on living donor transplantation.
Data was based on medical insurance claims which may not necessarily reflect the exact numbers, as there may be alternative sources of payment.
Conclusion
There is no RCT to date to inform the need of pre-transplant CHD testing in patients with CKD. This study adds to the growing epidemiological data which, despite many efforts, have failed to demonstrate any advantage of additional CHD testing in low risk and asymptomatic patients prior to the definitive treatment of kidney failure: a kidney transplant.
Summary prepared by
Arrsh Bajaj, MBBS
Department Of Internal Medicine,
St. Elizabeth Youngstown Hospital, Ohio, USA
And
Jeyakumar Meyyappan, DM,
Department Of Nephrology,
Sanjay Gandhi Postgraduate Institute of Medical Sciences,
Lucknow, India
NSMC Interns, Class of 2023