#NephJC Chat
Tuesday April 7, 2020 at 9 pm Eastern Daylight Time
Wednesday April 8, 2020 at 9 pm Indian Standard Time
Wednesday April 8, 2020 at 9 pm British Summer Time
Clin J Am Soc Nephrol 15 (2), 209-218, 2020 Feb 7
Randomized, Controlled Trial of Tacrolimus and Prednisolone Monotherapy for Adults With De Novo Minimal Change Disease: A Multicenter, Randomized, Controlled Trial
Nicholas Rhys Medjeral-Thomas, Christopher Lawrence, Marie Condon, Bhrigu Sood, Paul Warwicker, Heather Brown, James Pattison, Sunil Bhandari, Jonathan Barratt, Neil Turner , H Terence Cook, Jeremy B Levy, Liz Lightstone, Charles Pusey, Jack Galliford, Thomas D Cairns, Megan Griffith
PMID: 31953303
Introduction
Minimal change disease (MCD) is the most common cause of nephrotic syndrome in children and an important cause in adults as well (accounting for 10-25%). The goal of treatment is to achieve remission and the cornerstone for decades has been steroids. Left untreated, nephrotic syndrome is associated with potentially fatal complications like thromboembolism and infection, fortunately which have become increasingly rare with steroid treatment.
Data for steroid use is largely extrapolated from RCTs in children. About a third of people with MCD do undergo spontaneous remission, but usually after a prolonged period (Mak et al NDT, 1996). Over 80% of adults with MCD are steroid responsive. 50-70% of adult patients will experience relapse and up to a third of patients may become frequent relapsers or steroid dependent (Waldman et al, cJASN 2007). Similar data were reported in this large case series by Szeto et al (AJKD, 2015) and covered in this blog, which also pointed out the adverse effects. Though, steroids are generally well tolerated, drug related adverse effects (weight gain, diabetes mellitus, infection, osteoporosis, avascular necrosis) are common with prolonged or repeated courses.
This leads us to steroid-sparing drugs: what can we use that works for MCD, without the side effects that come with steroids?
Current KDIGO guidelines favour steroid use as initial treatment in MCD, reserving other immunosuppressive agents for frequently relapsing or steroid resistance cases. Alkylating agents such as cyclophosphamide have been used in MCD since the 1970s, inducing remission in frequent relapsing or steroid resistant adults (Uldall et al, Lancet 1972). Calcineurin inhibitors (CNIs), mycophenolate mofetil and now rituximab have also been used as documented in sporadic case reports, case series, and observational studies. Hogan et al (JASN 2013) provided a solid review of this data. From here on, we will focus on the CNI, tacrolimus, the drug investigated in the MINTAC trial.
T cell activation plays a crucial role in the pathogenesis of glomerular diseases. Calcineurin inhibition in T cells leads to a decreased production of cytokines such as IL2 and IFN-gamma. Antiproteinuric effects of CNIs are often attributed to its immunosuppressive action. However, CNIs also have direct effects on the cytoskeleton (and therefore shape) of podocytes (Zhang et al, Am J Nephrol 2012, Faul et al, Nat Medicine 2008).
Both cyclosporine and tacrolimus are used in MCD, often in patients that are either frequent relapsers, steroid-dependent, or steroid-resistant. Numerous observational studies have reported remission rates of 70-90% with cyclosporine (Waldman et al, CJASN 2007; Meyrier et al, Clin Nephrol 1991). A small case series suggests tacrolimus may have similar efficacy (Westhoff et al, Clin Nephrol, 2006). However tacrolimus shows more potent cytokine suppression and seems to cause less toxicity than cyclosporine, at least from the transplant literature (Denton et al, Lancet 1999). When compared to cyclophosphamide in steroid dependent patients, similar response rates were achieved at 24 months in this prospective cohort study by Li et al (NDT 2008). All patients in the study were able to discontinue steroids.
In the pediatric population, with steroid resistant MCD, tacrolimus was reported to be superior to cyclophosphamide (Gulati et al, Kidney Int 2012) and may have similar efficacy to cyclosporine (Sinha et al, NDT 2006). In one case report of steroid and cyclosporine resistant MCD, tacrolimus use was associated with remission.
For steroid-sparing treatments in the initial presentation of MCD, experience is limited. The table below gives a summary of published response rates for the initial episode of adults with MCD.
Source: Hogan et al, JASN May 2013, 24 (5) 702-711
Thus evidence remains sparse in tacrolimus monotherapy use, which leads us to the MinTac trial.
The Study
Methods
This study was a multicenter, prospective, open-label, randomized, controlled trial recruiting from 6 hospitals across the United Kingdom.
Funding: National Institute for Health Research Imperial Biomedical Research Centre.
Inclusion criteria:
Age >18 years
Nephrotic syndrome (defined as urine protein to creatinine ratio > 100 mg/mmol, approximately 1 g protein /g creatinine) and serum albumin < 3.0 g/dl.
New diagnosis of minimal change disease. Biopsy samples were examined at local nephrology units and if there was diagnostic uncertainty, they were reviewed by a single histopathologist.
Exclusion criteria:
Blood borne virus (hepatitis B, C or HIV infection)
Active infection
Previous treatment with immunosuppression (in past 18 months)
Pregnant or breastfeeding women.
Hypertension was treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and hypercholesterolemia with statins. If serum albumin was < 2.0 g/dl, subcutaneous low-molecular-weight heparin was used as prophylaxis, or aspirin 75mg if serum albumin was > 2.0 g/dl. During immunosuppressive treatment, participants at high risk of latent tuberculosis were treated with isoniazid 150mg and pyridoxine.
Intervention:
Eligible participants were randomized at a 1:1 ratio to receive either oral tacrolimus or prednisolone.
The tacrolimus arm received an initial dose of 0.05 mg/kg twice daily, with target trough levels of 6-8 ng/ml. At week 8, if inadequate clinical response, target blood trough level was increased to 9-12 ng/ml.
12 weeks after achieving complete remission, tacrolimus doses were titrated down over 8 weeks and stopped.
Participants in prednisolone arm received an initial dose of 1mg/kg per day with a maximum dose of 60 mg/kg per day.
One week after achieving complete remission, the steroid dose was halved for 4-6 weeks then gradually reduced and stopped over a further 6 weeks. Patients received a minimum of 16 weeks prednisolone. Bone and gastric protection was with calcium carbonate/cholecalciferol (1000mg/800IU) two tablets daily and omeprazole 20mg daily.
Analysis and Outcomes:
The primary outcome was the number of patients achieving complete remission of nephrotic syndrome at 8 weeks. Patients who achieved complete remission were followed up for 78 ± 2 weeks from complete remission or until relapse.
Secondary outcomes:
Proportion of patients achieving complete response at 16 weeks and 26 weeks
Proportion of patients who relapsed after complete remission
Change in serum creatinine from baseline
Rates of adverse events
Outcome Definitions:
Complete remission was defined as uPCR <50 mg/mmol.
Partial remission was defined as uPCR reduction of ≥ 50% from enrollment and a value between 50-300 mg/mmol.
Relapse defined as uPCR >300 mg/mmol in patients who achieved complete remission
Statistical Analysis
This was a non-inferiority (NI) trial. For more on the design on these trials, read the excellent explainer here from Manasi Bapat. The lower bound of the two-sided 95% confidence interval was set as 10% (Δ value of -0.1). For non-inferiority trials, the choice of delta is justified on clinical and statistical grounds. Allowing for a dropout rate of 10%, the investigators calculated 52 patients were required. The expected complete remission rate for patients treated at 8 weeks was estimated 60% (n= 23) and 84% (n=39) in prednisolone and tacrolimus groups respectively. Though this was not specified in the methods, the authors do provide both per-protocol and intention to treat analyses (ITT). For NI trials, in general (though with some exceptions), the per-protocol analysis can be more robust.
Results
50 patients with de novo disease were included in final results (Figure 1). 25 patients randomised to tacrolimus treatment and 25 to prednisolone arm.
Figure 1 from Rhys Medjeral-Thomas et al, CJASN 2020
The table 1 depicts the baseline characteristics of patients. They were mostly young (mean age ~ 40 years) and with well preserved kidney function. 40% (n=20) of patients were on antihypertensive treatment in the form of ACEi/ARB.
Table 1 from Rhys Medjeral-Thomas et al, CJASN 2020
Primary Outcome
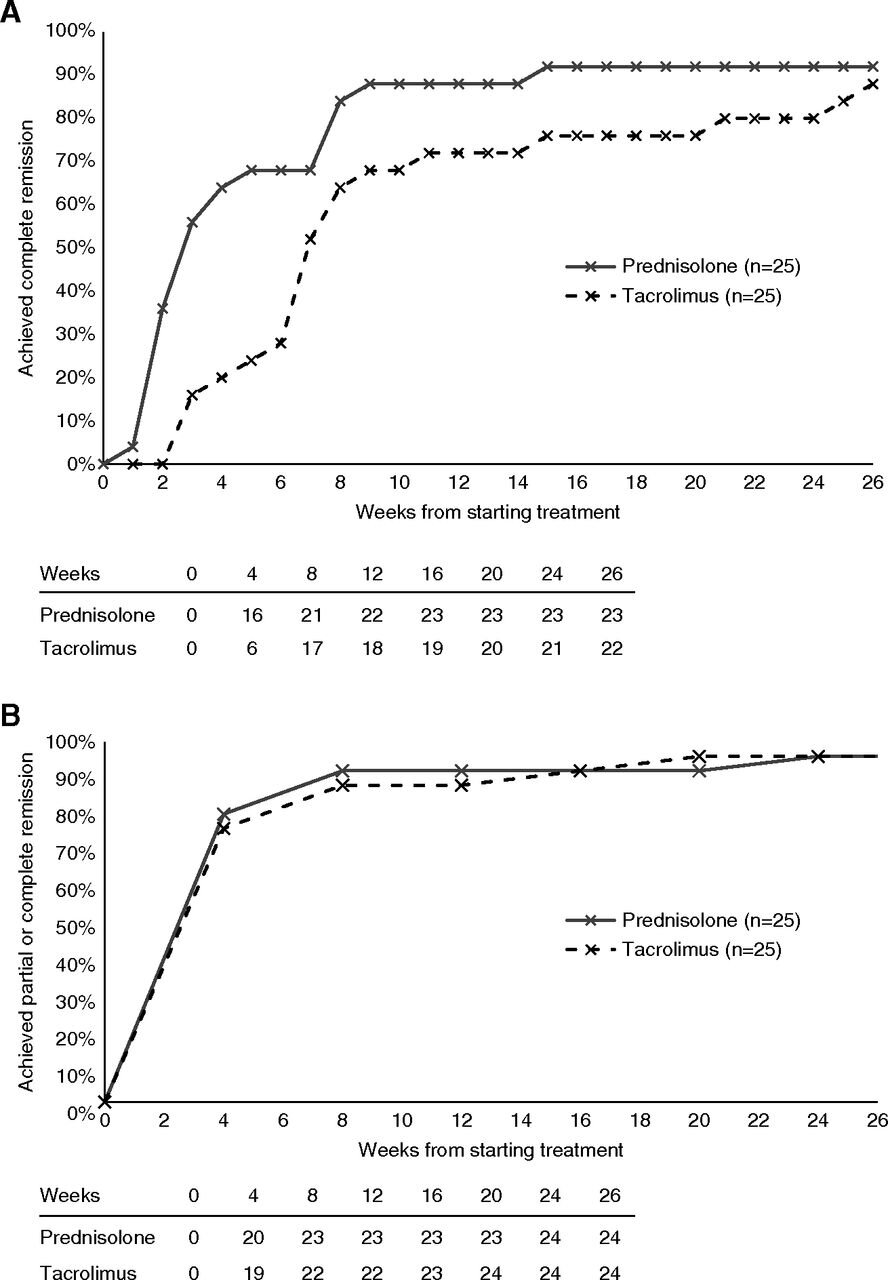
At 8 weeks, there was no difference in proportion of patients that achieved complete remission in both treatment groups, 84% with steroids and 68% with tacrolimus (p = 0.32) in the per-protocol analysis.
Figure 2 from Rhys Medjeral-Thomas et al, CJASN 2020, shows per-protocol analysis
But this was a non-inferiority trial. For NI to be established, tacrolimus had to be within 10% of steroids for complete remission at 8 weeks. The difference here is 16% (95% CI, -11% to 40%), meaning non-inferiority was not shown. For the ITT analysis, the difference in remission rates was 21% (95% CI, −6% to 44%), again not non-inferior. Since the 95% CI straddles across the 10% NI margin, these results do not establish the inferiority of tacrolimus, nor do they establish its non-inferiority. See below for what this represents
Adapted from Schumi and Wittes, overlaying present results. Not to scale
Secondary Outcomes
These are presented in table 2. These are all per-protocol numbers, see table below for ITT and how they compare (not very different). As can be seen, the difference between tacrolimus and steroids, which seems large at 4 weeks, is minimal at 26 weeks. Of interest, the change in creatinine (given potential tacrolimus nephrotoxicity) was also not different between groups.
Table 2 from Rhys Medjeral-Thomas et al, CJASN 2020, shows per-protocol analysis
For how the per protocol stacks up against the ITT analysis, the authors do provide granular details, which we have tabulated below:
Unsurprisingly, mean serum albumin was significantly higher in the pred group than the tac group at 4 weeks (3.24g/dl vs 2.75g/dl, p = 0.03). No significant differences detected beyond 4 weeks. Differences in uPCR did not reach statistical significance at any point during follow-up. Median uPCR in pred group, 11 mg/g (IQR 6-109 mg/g) and tac group, 58mg/g (IQR 6-216 mg/g).
Relapse rates
There was no significant difference in relapse between prednisolone and tacrolimus groups. Patients were followed up for more than 1 year and time to relapses were similar in both treatment groups (Figure 3, below). When relapses occurred, over half patients had stopped immunosuppressants (53% in pred group vs 56% in tac group). Median time of complete remission to relapse was 22 weeks in prednisolone cohort and 32.7 weeks in tacrolimus cohort (p =0.72).
Figure 3 from Rhys Medjeral-Thomas et al, CJASN 2020
Adverse Events
We are probably all familiar with the common side effects of prednisolone and tacrolimus. Table 3 gives the safety profile of the study, which is comparable in both treatment groups. Most frequently reported adverse events were chest infections (requiring antibiotics).
Table 3 from Rhys Medjeral-Thomas et al, CJASN 2020
Discussion
Imagine a world in MCD without corticosteroids. This study is the first prospective, multicenter randomised trial to explore tacrolimus monotherapy. The results suggest tacrolimus is an effective alternative treatment to steroids for de novo minimal change disease in adults. Though tacrolimus did not show inferiority at 8 weeks, there was little difference by 26 weeks (difference in remission 4%; 95% CI, −17% to 25%, still straddling the 10% NI margin). Relapse rates were also comparable in both treatment arms. Despite not being able to demonstrate non-inferiority, the data on the effects of tacrolimus monotherapy in MCD are useful for people looking at using this.
Limitations
This was a 52 patient trial, could a larger sample size have had a different result? It would have given greater precision for sure, but would this have clustered around the point estimate of -16% resulting in inferiority, or moved into the solidly NI territory is hard to say. Adult MCD is not very common, so this still represents the best quality of data we have so far.
Conclusions
Will this study influence your practice? The majority in the nephrology community would agree to avoid repeated and/or long courses of steroid use due to adverse effects and comorbidities associated. Patients also find the cosmetic side effects of steroids unappealing. If concerns are exposure to steroids, what about using tacrolimus in combination with low-dose/short course steroid to induce remission? This approach was investigated in a small pilot study of 14 patients; low-dose prednisolone (0.5 mg/kg/day) + tacrolimus inducing complete remission (71.3% of patients by 4 weeks, and 100% of patients by 20 weeks after therapy). In another larger trial (Li et al, JASN 2017), tacrolimus monotherapy following IV methylprednisolone at (0.8 mg/kg per day) for 10 days was non-inferior to conventional steroids, and did show a lower relapse rate compared to the present study.
Can we say goodbye to steroids for good? Given the results in this study, we should certainly consider tacrolimus as initial therapy in select patients. Perhaps this is especially relevant in patients with relative contraindications to high dose steroid use such severe osteoporosis, steroid-induced psychosis, uncontrolled diabetes or morbid obesity. Like in other glomerular diseases, the monoclonal antibodies are coming (or have already arrived). B cell depleting therapy is used more readily than ever; however, the MinTac trial demonstrates a role for tacrolimus.
Summary by Renua Aiyegbusi, Renal Registrar, Glasgow
NSMC graduate, Class of 2019