#NephJC Chat
Tuesday, August 22nd, 2023 at 9 pm Eastern
Wednesday, August 23rd, 2023 at 9 pm Indian Standard Time and 3:30 pm GMT
Identification and validation of urinary CXCL9 as a biomarker for diagnosis of acute interstitial nephritis
Dennis G Moledina, Wassim Obeid, Rex N Smith, Ivy Rosales, Meghan E Sise, Gilbert Moeckel, Michael Kashgarian, Michael Kuperman, Kirk N Campbell, Sean Lefferts, Kristin Meliambro, Markus Bitzer, Mark A Perazella, Randy L Luciano, Jordan S Pober, Lloyd G Cantley, Robert B Colvin, F Perry Wilson, Chirag R Parikh
PMID: 37395276
Introduction
Making the clinical diagnosis of acute interstitial nephritis AIN’t easy. An indolently rising creatinine in a hospitalized patient with known exposure to many different drug classes, can present quite a diagnostic dilemma. Around 20% of patients who undergo kidney biopsies for acute kidney injury (AKI) have acute interstitial nephritis (AIN) (Haas M et al, AJKD 2000). The true incidence of AIN, however, might be quite different as many patients get diagnosed clinically without the assistance of definitive urine or serum diagnostic markers. In this study, Moledina DG et al identify and validate CXCL9 (chemokine C-X-C motif ligand 9) as a biomarker tool to help make the clinical diagnosis of AIN.
What is the role of CXCL9 in tubulointerstitial inflammation? CXCL9 primarily attracts T-lymphocytes by binding to CXCR3, a G-coupled protein receptor (Whitting D et al, J Immunol 2004). CXCR3 expression is upregulated by interferon gamma and correlates with tissue infiltration by T-lymphocytes. CXCL9 plays a role in kidney allograft rejection and has been validated as a risk-stratifying biomarker in this domain (Hricik DE et al, AJT 2013). Furthermore, it seems to be restricted to the tubulointerstitial compartment, making it a potentially great marker for AIN (Jackson JA et al, AJT 2011). Here is a helpful infographic describing the role of CXCL9 by our very own @NephroSeeker.
Medications have been implicated in 75% of AIN cases while systemic diseases, infections, and idiopathic causes account for the other quarter (Praga M et al, Kidney Int 2010). AIN has classically been taught to present with a triad of fever, rash, and eosinophilia/eosinophiluria in the setting of AKI. However, although AKI of varying degrees is present in all patients with AIN (Clarkson MR et al, Nephrol Dial Transplant 2004), a case series found that less than 20% presented with any systemic symptoms, and only 7% presented with the classic triad (Muriithi AK et al, AJKD 2014). Urine studies are typically bland, but may show leukocyturia or WBC casts (Praga M et al, Kidney Int 2010). Historically, eosinophiluria has been associated with AIN, and although ordered frequently, has no apparent value in confirming the diagnosis of AIN (Lusica ML et al, JHM 2017). In a 1985 study, urine eosinophils were only detected in 9 out of 65 patients diagnosed with AIN by biopsy or clinical criteria. In addition, urine eosinophils were associated with a variety of other medical conditions (Corwin HL et al, Arch Intern Med 1985). Furthermore, a 2013 study found that the positive and negative predictive values of eosinophiluria were only 15% and 85%, respectively (Muriithi AK et al, CJASN 2013). Hence, urine eosinophils are of little value clinically, and a kidney biopsy remains the gold standard for the diagnosis of AIN. Biopsy specimens typically show interstitial edema, cellular infiltrates, and fibrotic changes - as described in a 2019 review by Dr. Kammi Henriksen from the Renal Fellow Network. Clinicians do sometimes defer biopsies, due to risk of bleeding or severity of illness, and empirically treat with steroids (Sanchez-Alamo B et al, Nephron 2023). Having a sensitive and specific biomarker would be very useful, allowing clinicians to potentially diagnose and treat AIN without the need for a biopsy. Finally, a recent study found that the utilization of urinary TNF-alpha and IL-9 levels improved discrimination of AIN from other causes of AKI (Moledina DG et al, JCI Insight 2019). This same work group proposed an additional biomarker, CXCL9. This study delves into how CXCL9 was identified as a potential marker of AIN by the use of urine proteomics and biopsy proven validation cohorts.
The Study
Design/Study Population:
This study utilized both a discovery and external validation cohorts. The primary purpose of the discovery cohort was to identify potential biomarkers in biopsy-proven AIN patients, and to determine diagnostic thresholds for said biomarkers. The external validation cohort was then used to statistically evaluate diagnostic thresholds.
Here is a good infographic summarizing the discovery and external cohorts and measured outcomes of this study:
CXCL9 was measured by sandwich immunoassays. Cox KL et al does a great job at explaining sandwich immunoassays, as depicted below.
Diagram of a sandwich ELISA. The addition of the enzyme’s substrate leads to color development. The amount of color (absorbance) is directly proportional to the analyte concentration. From: Cox KL et al, The Assay Guidance Manual, 2019.
Statistics
Urine Proteomics: Only proteins that were present in at least 75% of the samples were shown. These proteins were plotted on a Volcano chart with the y-axis having p-values (log transformed) and the x-axis having fold differences (log transformed). The latter was measured by comparing the differences between cases and controls. As you can see in Figure 1, CXCL9 stands out as a potential marker.
Figure 1: Volcano plot demonstrating associations of aptamer-based measurement of urine proteins with acute interstitial nephritis diagnosis. From: Moledina DG et al, JCI, 2023.
Urine Biomarker: All CXCL9 levels were normalized to urine creatinine. The independent association of CXCL9 and biopsy proven AIN was done through logistic regression and reported as an odds ratio. The thresholds were divided into 4 quartiles, and the top two were compared with the bottom one. Model 1 looked at only CXCL9 and AIN. Model 2 looked at the association of AIN and the clinical nephrologists’ prebiopsy suspicion of AIN (yes/no). Model 3 looked at the association of AIN and the AIN diagnostic index, which included serum creatinine, serum BUN to creatinine ratio, urine specific gravity, and urine protein. The AIN diagnostic index is a validated tool for the histological diagnosis of AIN (Moledina DG et al, Nephro Dial Transplant 2022). All of the above models were adjusted for demographics (age, sex, race), comorbidities (diabetes and hypertension), plasma CXCL9 (log transformed), and urine IL-9 and TNF-alpha (both log transformed). AUC was produced for all 3 models mentioned above. Afterwards, they added CXCL9 to model 2 and 3 to look at the change in AUC.
Optimal Biomarker Combinations: The authors used least absolute shrinkage and selection operator (LASSO) in which all 16 biomarkers of this cohort were included in 1000 iterations of randomly generated subsets consisting of 70% of the discovery cohort, to see which were most associated with AIN. A logistic regression was then created with these biomarkers and then later applied to the remaining 30% of the discovery cohort, as well as external validation cohorts. AUCs were created for the remaining 30% of the discovery cohort and external validation cohorts. As the proportion of participants with AIN among those with AKI had been noted to be between 10% and 20%, the authors showed test characteristics at two AIN probability cutoffs (10% and 20%) with CXCL9 alone, and with all 3 biomarkers. They also applied multiple imputations to account for missing data.
Funding
This study was primarily funded by the National Institute of Diabetes and Digestive and Kidney diseases. Additional funding was provided by the Yale O’Brien Center, which also provided biopsy samples for the discovery cohort.
In our quest for biomarkers in Nephrology, let’s see how CXCL9 performed in the diagnosis of AIN.
1. Can CXCL9 distinguish between AIN and other AKI causes?
2. Did CXCL9 correlate with the severity of AIN?
3. Did CXCL9 perform the same between discovery and validation cohorts?
4. Can CXCL9 help replace renal biopsies in clinical practice?
Let’s see if we can gAIN any insights to these questions, and examine the data.
Results
Discovery Cohort
Eighty eight participants were included in urine proteomic analysis, which included 31 (35%) participants with biopsy-confirmed, pathologist-adjudicated AIN and 57 (65%) with a random spectrum of other diagnoses as controls (Figure S3 and Table S1).
Figure S3. STARD flow diagram. From: Moledina DG et al, JCI, 2023.
Table S1. Histological diagnosis among participants included for urine proteomics analysis. From: Moledina DG et al, JCI, 2023.
These participants had comparable histological features on biopsy. Those patients with AIN, however, tended to have lower urine albumin-to-creatinine ratios and higher serum creatinine levels (Table S2).
Table S2: Baseline characteristics of participants who underwent urine proteomics. From: Moledina DG et al, JCI, 2023.
Urinary CXCL9 Correlated with AIN Severity
There was a strong correlation between urine proteomics and immunoassay measurements of CXCL9 (correlation value = 0.99). Participants with higher CXCL9 levels were more likely to be older, have AKI that required dialysis, as well as higher serum creatinines at biopsy, and higher urine albumin-to-creatinine ratios.
CXCL9 levels were 5.5 fold higher in patients with AIN compared to those in the control group, regardless of the criteria used to define AIN. Median CXCL9 levels were 8-fold higher in those with AIN when compared to those with acute tubular injury (ATI) (AIN versus ATI, 60.3 [16.4, 1103.4] versus 7.7 [3.3, 28.7]; P = 0.0001).
Table 1: Participant characteristics by C-X-C motif ligand 9 (CXCL9) quartiles. From: Moledina DG et al, JCI, 2023.
CXCL9 was linked to the severity of AIN interstitial characteristics such as interstitial infiltration and tubulitis, but not to the degree of interstitial eosinophilia (who would have guessed that?!), tubular damage or glomerular crescents.
Figure S7. Association of urine CXCL9 with severity of histological features. Non-parametric trend test; severity of interstitial features as determined by median value of three adjudicating pathologists. From: Moledina DG et al, JCI, 2023.
Figure 2: Urine CXCL9 levels are higher in acute interstitial nephritis compared with controls in the discovery cohort. From: Moledina DG et al, JCI, 2023.
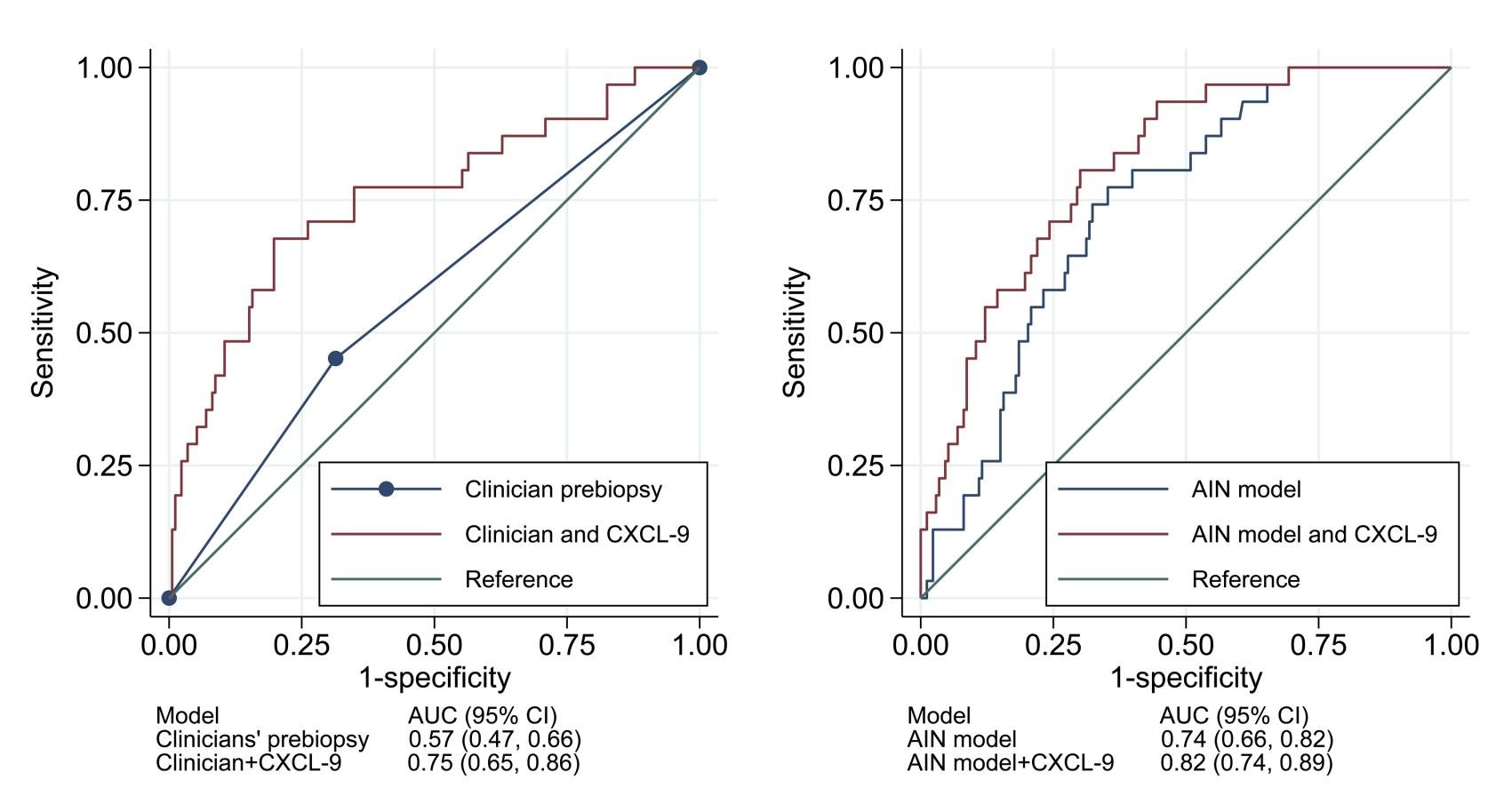
Urinary CXCL9 was independently associated with AIN diagnosis and improved the AUC for AIN diagnosis.
The authors reported odds ratios (and 95% CIs) for AIN diagnosis per doubling of CXCL9 as well as for the two highest quartiles using the lowest quartile as the reference group (Model 1).
Model 1 investigated the univariable association of CXCL9 with AIN.
Model 2 was controlled for the clinical nephrologists’ prebiopsy suspicion of AIN.
Model 3 controlled for AIN diagnostic index, a recently developed model of 4 clinically available variables (serum creatinine, serum blood urea nitrogen /creatinine ratio, dipstick urine specific gravity, and dipstick proteinuria) that was validated for histological AIN diagnosis by Moledina et al .
The odds of AIN was 40% higher with doubling of values for CXCL9 (OR 1.4; 95% CI: 1.2–1.5). Compared to values in the lowest quartile, those in the highest quartile had 6-fold higher risk of AIN (OR 6.0; 95% CI: 1.8–19.9, Model 1). The authors noted similar results in multivariable analyses controlling for clinicians’ prebiopsy suspicion for AIN (Table 3, Model 2) and for an externally validated statistical model for AIN (Table 3, Model 3). Addition of CXCL9 improved the AUC, over clinicians’ prebiopsy impressions, by 0.18 to 0.75 (95% CI: 0.65–0.86). The AUC of CXCL9 for differentiating AIN from ATI was 0.77 (0.66, 0.88).
Figure 3: Urine CXCL9 improved the AUC for acute interstitial nephritis compared with existing information. From: Moledina DG et al, JCI, 2023.
External Validation Cohorts
The authors validated their findings in two external cohorts with histologically confirmed diagnosis, C-PROBE (n = 12; AIN = 4) and Icahn School of Medicine (n = 21; AIN = 6). The levels of CXCL9 were found to be higher in patients with AIN compared to the control group. CXCL9 had an AUC of 0.94 (95% CI: 0.86–1.00) for AIN diagnosis in the external validation cohorts.
Table S6. Baseline characteristics of participants in external validation cohorts. From: Moledina DG et al, JCI, 2023.
Figure S9. CXCL9 levels were higher in patients with AIN than in controls in each external validation cohort. From: Moledina DG et al, JCI, 2023.
Urinary CXCL9 test characteristics for AIN diagnosis
The test characteristics of urinary CXCL9 in the discovery and external validation cohorts were at four cut-points derived from the discovery cohort: the 25th, 50th, and 75th percentiles, and a cut-point derived by maximizing the sum of sensitivity and specificity using the Youden index. At the 75th percentile cut-point (58.9 ng/g), the authors noted specificities of 79% and 100% and positive predictive values of 30% and 100%, respectively, in the discovery and validation cohorts.
Table 4: Test characteristics of urine CXCL9 for AIN diagnosis in discovery and external validation cohorts. From: Moledina DG et al, JCI, 2023.
Patients with AIN had higher kidney tissue mRNA expression of CXCL9
The authors also found that there was higher tissue mRNA expression of CXCL9 in biopsies from patients with AIN than in biopsies from patients with non-AIN etiology in the control group.
Figure 5: CXCL9 expression in kidney biopsies was higher in acute interstitial nephritis than in controls. From: Moledina DG et al, JCI, 2023.
Association of CXCL10 with AIN
CXCL10 is also induced by IFN-γ and binds to their shared receptor, CXCR3. However, when controlled for CXCL9, the association of CXCL10 with AIN was no longer significant (adjusted OR, 0.95 [0.75, 1.22]), whereas the association of CXCL9 with AIN was found to be independent of CXCL10 levels.
Optimal Combination of biomarkers
In order to determine the optimal biomarker combination for the diagnosis of AIN, LASSO feature selection algorithm was used. It was noted that IL-9, TNF-α, and CXCL9 were selected in over 75% of models. The authors trained a logistic model for these 3 biomarkers for the outcome of AIN on the first 70% of discovery cohort participants (training set) and applied these model weights to the next 30% of discovery cohort participants (test set) and the external validation cohorts.
Figure S10: Urine biomarker selection using LASSO feature selection algorithm. From: Moledina DG et al, JCI, 2023.
An AUC of 0.89 (95% CI: 0.77–0.98) in the test set and 0.87 (95% CI: 0.70–0.99) in the external validation cohort was noted. At a model probability cutoff of 10%, the sensitivity and specificity were found to be 87% and 60%, respectively, in the test set. Similarly, in the external validation set for the model containing all 3 biomarkers, sensitivity and specificity were 90% and 70%, respectively. From the precision recall curves, the combination of urinary CXCL9 together with TNF-α and IL-9 proved to be the optimal combination of biomarkers for AIN diagnosis.
Figure S11. Receiver operating characteristics analysis of the 3-biomarker model in the test and external validation sets. From: Moledina DG et al, JCI, 2023.
Discussion
It has been known for some time that urine and serum eosinophils lack the specificity and sensitivity to be used as reliable diagnostic markers for AIN. In addition, many clinicians may be reluctant to perform invasive kidney biopsies in patients with less severe AKI (not requiring dialysis), due to risk-benefit analysis. Also, in those patients in whom the diagnosis is in question, antiplatelets agents or anticoagulants may delay performing a definitive renal biopsy. The search for a reliable serum or urine biomarker has been an ongoing process for more than a decade. In this study, urinary CXCL9 was identified and validated as a diagnostic biomarker for AIN.
Key Findings:
IFN-𝛾 is a significant upstream regulator of the inflammatory changes seen in AIN using both urine proteomics and tissue transcriptomics. This should prompt research into IFN-targeting treatments for the treatment of AIN.
CXCL9 levels were over 100 times higher than IL-9 and TNF- levels, which is consistent with the fact that chemokine concentrations are higher than cytokine concentrations when it comes to activating specific receptors.
Finally, the authors showed that urinary CXCL9, TNF-alpha and IL-9 turned out to be an optimal combination of biomarkers in the diagnosis of AIN, thus obviating the need for biopsy (in certain patients) to arrive at the diagnosis.
Acute kidney injury, caused by AIN, can be addressed by withdrawal of offending drugs and/or initiation of corticosteroid therapy. The presumptive diagnosis of AIN may have consequences including withdrawal of life-saving medications, such as antibiotics or anticancer drugs. In addition, corticosteroid therapy is associated with a wide range of serious side-effects. Hence, from the current study we understand that the urine biomarker CXCL9 has the potential to greatly improve clinical care. By using a non-invasive urine test for the diagnosis of AIN, treatment and clinical decision making may not have to be unnecessarily delayed. Although CXCL9 testing does not give clinicians the exact inciting medication or disease process that triggered AIN, more rapid and definitive diagnoses will lead to better algorithms for tracking down specific etiologies. Specific thresholds will need to be utilized to maximize sensitivity and specificity (see below). AgAIN, most nephrologists would not complAIN about gAINing a new era of certAINty in ascertAINing a no-brAINer diagnostic tool for AIN.
Figure 1: A framework that incorporates testing for urinary CXCL9 may improve the accuracy of diagnosis and management of AKI. From: Canney M et al, JCI 2023.
Strengths:
CXCL9 has the potential to be integrated into current platforms in clinical laboratories or created as a point-of-care test, which is significant given that the higher concentration of CXCL9 portends superior laboratory test features.
In actuality, point-of-care devices are under development that can identify acute cell mediated rejection, which is similar to AIN, where the tubulointerstitial immune process spares the glomeruli.
Additionally, the study findings shed light on the pathophysiology of AIN. Patients with ATI had very low levels of CXCL9 in comparison to the substantial elevation of CXCL9 in tissue and urine from AIN patients. In line with this Xu et al, demonstrated that despite T cell migration to the interstitial compartment, a recent investigation of the cellular events causing ATI in mouse models of ischemia-reperfusion injury showed essentially minimal increase of CXCL9 in the kidney (Xu L et al, Nat Commun 2022).
Limitations:
The authors did not investigate why the related IFN-induced chemokines CXCL10 or -11 do not share the same robust connection with interstitial inflammation as CXCL9 does. One possibility is that, as found in other organs, distinct cellular targets of IFN-𝛾 result in preferential production of one chemokine over another.
Conclusion
AIN is a common cause of AKI. Unfortunately, at present clinicians do not have definitive diagnostic tools for AIN other than invasive kidney biopsies. Kidney biopsies carry risks including bleeding, which is elevated in patients who are frail or on anticoagulants. These elements may delay diagnosis and treatment initiation. This study introduces a new urine biomarker, CXCL9, that may increase diagnostic precision and improve clinical care.
Summary prepared by
Melvin Chan
Pediatric Nephrology Fellow
University of Colorado
and
Subashri M
Assistant Professor
Department of Nephrology
Government Thoothukudi Medical College & Hospital
Tamilnadu, India
NSMC Interns, class of 2023
___________________________________________
Reviewed by: Brian Rifkin, Shweta Shah, Swapnil Hiremath, Cristina Popa