Preamble
As with all the other pages on COVID-19 here on NephJC, these are not meant to be treatment recommendations based on sound evidence. The data we have so far is mostly anecdotal: stories, a few case series, and the occasional published study (sometimes not peer reviewed). Our aim is to discuss the science of what we know; review the biology and mechanisms of the pathology; provide practical tips to treating acute kidney injury the middle of a pandemic. We hope these words help the reader make the decisions they need to make. These are the opinions from the workgroup, and we constantly update the page as data trickles in.
Before we start reviewing the literature it is important to recognize the current landscape of peer review (or lack thereof). Especially in the fast moving world of COVID-19 research. Brian Byrd provides a nice overview for our readers here. The differences between preprints, letters to editor, rapid response, and other publication types are discussed in detail.
Contributors
Content curated by
Steve Coca, DO, MS, Mt Sinai, New York NY
Swapnil Hiremath, MD, MPH, University of Ottawa, Canada
Jay Koyner. MD, University of Chicago, Chicago IL
Jennie Lin, MD, MTR, Northwestern University, Chicago IL
Roger Rodby, MD, Rush University, Chicago IL
Anitha Vijayan, MD Washington University in St. Louis, St. Louis MO
Paul Welling, MD, Johns Hopkins, Baltimore MD
Content Reviewed by
Linda Awdishu, Pharm. D, MAS, University of California San Diego, CA
Dan Batlle, MD, Northwestern University, Chicago IL
Manasi Bapat, MD, CA
Anna Burgner, MD, MEHP, Vanderbilt University, Nashville, TN
Edward Clark, MD, MSc, University of Ottawa, Canada Amanda Dijanic Zeidman, MD, Mt Sinai Hospital, New York, NY Michael Heung, MD, University of Michigan at Ann Arbor MI Raymond Hsu, MD, UCSF, CA
Kenar Jhaveri, MD, Hofstra-Northwell School of Medicine, NY Nikhil Shah, MBBS DNB University of Alberta, Edmonton, Canada. Matthew Sparks, MD Duke University, Durham NC Sinead Stoneman, MD, Cork, Ireland
Joel Topf, MD, Detroit, MI
Juan Carlos Q Velez, MD, Ochsner Health, New Orleans, LA
Date of Last Update: October 4th 12:57 Eastern
What was updated: update on remdesivir
Official and FOAMed Resources for additional reading
General COVID Management
The IBCC from Josh Farkas (@pulmcrit)
COVID Protocols (from Brigham & Women’s Hospital)
University of Washington Resource site
Handbook from the First Affiliated Hospital, Zhejiang University School of Medicine
UpToDate on COVID-19 (all open access for now)
Specific for AKI
COVID-19 and AKI FAQs
What is the risk of AKI in patients with COVID-19?
Data here is sketchy. Because of variable testing rates the denominator is uncertain. So keep that in mind when looking at the numbers.
The data on acute kidney injury (AKI) in patients with COVID-19 is summarized below (Table) and mostly represent patients who are hospitalized. We have tried to highlight which data is from all hospitalized patients (which shows a lower AKI incidence) and the ones from critically ill patients (with higher rates). Additionally, keep in mind that criteria for admission to critical care units vary from region to country, so your experience might differ.
From Zhou et al (Lancet 2020), AKI seems to develop at a median of 15 days (interquartile range 13 - 19.5 days), whereas Cheng et al (Lancet 2020) (which used the more sensitive KDIGO criteria) mentions that most AKI developed within 7 days of admission. From the recent data from New York (Hirsch et al, Kidney Int), AKI seems to develop soon after hospitalization, with a characteristic spike around the time of requiring intubation.
Figure 2 from Hirsch et al, Kidney Int, 2020
The N (number) for AKI in these manuscripts closely track the need for kidney replacement therapy (KRT), so this data often does represents severe AKI. More recent studies (marked with * below) seem to be using KDIGO AKI criteria. In studies that don’t mention how AKI was defined, we suspect this is mostly picking up severe AKI, with exceptions as noted.
With the above caveats, the incidence of AKI ranges from 0.5% to 39% in the studies from China. In Hong Kong, Chu et al (Kidney Int 2005) found an incidence of 7% in SARS. The AKI was mostly due to ATN, with the addition of a few cases of rhabdomyolysis. Most of these patients presented with normal kidney function (no underlying CKD). Is this higher than what one would expect from patients hospitalized with acute illnesses? Not really.
In an earlier version of the table, we had data that was from the same centre, and we believe the same dataset, which we have tried to exclude in this version. There still might be some duplication, so note the center and period of study in the columns below.
* Used KDIGO criteria for AKI definitions. And yes, we noticed that the Chen Lancet 2020 study shows a higher rate of CKRT than AKI. That is what the study reported. For more on strange AKI risk from the Wang study from Am J Nephrol, and whether the data is correct, check this twitter thread. Bold data refers to incidence in critically ill patients. We have deleted rows of studies from the earlier version of this table which were clearly from same institution and period of time (to avoid double counting), however the data from the first 3 studies from Jinyintan Hospital likely still does represent overlapping data
Sources: Guan et al, NEJM; Wang et al, JAMA; Chen et al, Lancet; Zhang et al, MedRXiv; Diao et al, MedArXiv; Zhou at al, Lancet; Cheng et al KI; Wang et al AM J Nephrol; Yang et al Lancet Resp Med; Xiao et al MedRXiv; Cao et al MedRXiv; Liu et al Sci China Life Sci; Wu et al Clin Inf Dis; Yang et al J Infect;
Bold data refers to incidence in critically ill patients.
Source: Arentz et al, JAMA; Hirsch et al, Kidney Int; Chan et al, MedRXiv; ICNARC dataset (accessed May 8); Mohamed et al, Kidney360
AKI and KRT in the Critically ill
* of ventilated patients
Source: Hirsch et al, Kidney Int; Chan et al, MedRXiv; ICNARC dataset (updated May 29); Mohamed et al, Kidney360; Cummings et al, Lancet
Thus, in the critically ill patients, the incidence of AKI is > 50 %, and the need for KRT of all patients admitted to a critical care unit is at least 25 %. Much higher than earlier estimates from China.
Also note that some case series do not report any data on AKI. eg McMichael et al, NEJM and Graselli et al JAMA. We consider that these is unfortunate, and among critically ill patients, the incidence of needing KRT might be close to 15-25%, thus a necessary component of any published report.
What are the causes and characteristics of AKI in these patients?
So far, the majority of AKI in patients with COVID-19 seems to be acute tubular injury (ATI) in the setting of multiorgan failure but not necessarily shock. Some report of rhabdomyolysis exist, there is a suspicion of microthrombosis, and other anecdotes are of a hypercatabolic state. A study from New Orleans (Mohamed et al, Kidney 360, 2020) does a thorough job of ascertaining AKI causes, with acute tubular injury being the predominant contributor as seen here:
Table from Mohamed et al, Kidney 360
While more data is expected in the coming days to weeks, let’s review the existing pathology findings.
In terms of histopathological findings, more tissue data are becoming available. A postmortem case series study (Su et al. Kidney Int, 2020) reported that 9 out of 26 autopsied COVID-19 patients in China experienced AKI, primarily characterized on light microscopy by diffuse proximal tubule injury and some frank necrosis (see figure below).
Su et al., Kidney International, 2020. Proximal tubules with loss of brush border (a) and vacuolar degeneration indicated by arrows (b), with necrotic epithelial debris in tubular lumens (asterisk). Erythrocyte aggregates were often seen obstructing peritubular capillaries (arrowhead).
Another preprint (Diao et al, MedRXiv, 2020) of 85 patients who had kidney function details available reports on the autopsies from 6 patients who died. The kidney tissue, on light microscopy, mostly showed severe acute tubular necrosis with CD68+ macrophage infiltration of the tubulointerstitium. Some immune cell infiltration was also seen in Su et al.’s report. C5b-9 deposition on tubules was observed in all six cases, although very little deposition was seen in glomeruli and capillaries.
Diao et al., medRXIV, 2020. (A) CD68+ cells (macrophages) are seen in COVID-19 cases but not in normal healthy kidney tissue. Some CD8+ T Cells and CD56+ (natural killer) cells are also seen in disease cases. (B) C5b-9 staining is most prominent in tubules of disease cases and is only faintly detected in glomeruli and capillaries. No staining is observed in normal healthy control kidney tissue.
From the 2005 SARS study, a small risk of rhabdomyolysis was reported as contributing to AKI events. Su et al. also reported presence of pigmented casts containing high levels of creatine phosphokinase, consistent with rhabdomyolysis, in 3 out of the 9 cases. An example is shown in panel f below.
Source: Su et al., Kidney International, 2020.
COVID-19 and Collapsing Glomerulopathy
Initially most of the histopathological data on COVID-19 related AKI came from China without diverse ancestral backgrounds represented, but more kidney biopsy series have emerged from other nations with patients of different ancestral backgrounds. One of the earliest case reports from the United States (Larsen et al. KI Reports 2020) revealed that an African American COVID-19 patient with high-risk APOL1 genotype (G1/G1) and respiratory distress developed severe collapsing glomerulopathy that was non-oliguric with a spot urine protein/Cr ratio of 25 mg/g. Although her respiratory status improved, the patient became dialysis-dependent during her hospital course and had not recovered kidney function at the time of discharge. Her biopsy findings below revealed tubular injury (panel A), collapsed glomerular tuft with epithelial hyperplasia (panel B), foot process effacement (panel C), and tubuloreticular inclusions within the glomerular endothelial cell (panel D).
Although it is unclear whether the patient did have some baseline level of APOL1 nephropathy prior to her SARS-CoV-2 infection, cases of potential APOL1-mediated collapsing glomerulopathy triggered by SARS-CoV-2 have been reported. Wu et al. published a biopsy series of six Black COVID-19 patients with AKI (mean serum creatinine 6.5 mg/dL, mean urine protein-creatinine ratio 11.5 g); all six patients had a high-risk APOL1 genotype. Five out of these six patients required hemodialysis. Involvement of APOL1 risk alleles in collapsing glomerulopathy was also seen in some New York cases (Kudose et al. JASN 2020, Peleg et al. KI Reports 2020).
In France, two COVID-19 patients of African ancestry and with the high-risk APOL1 genotype (G1/G, G1/G2) developed collapsing focal segmental glomerulosclerosis (FSGS) with mild interstitial fibrosis and a widely dispersed mononuclear inflammatory infiltrate. The infiltrate for one of the patients was further characterized and stained positive for CD68+ macrophages (Couturier et al. Clinical Kidney Journal 2020). Interestingly, another report from France (Lazareth et al. AJKD 2020) documented collapsing glomerulopathy in an African-ancestry kidney transplant recipient with COVID-19. The patient’s allograft biopsy revealed that the donor carried one APOL1 risk allele (G0/G2), which is considered a low-risk genotype, and the patient had the low-risk G0/G0 genotype.
Other Pathologic Features
In addition to ATI, ATN, and collapsing glomerulopathy on an APOL1 risk allele background, other biopsied COVID-19 patients had findings consistent with membranous glomerulopathy, anti-GBM nephritis, exacerbation of preexisting autoimmune GN, and allograft rejection (Kudose et al. JASN 2020). Another biopsy series from New York (Sharma et al. JASN 2020) also revealed diverse glomerular findings on top of ATI, and two patients in the series were found to have thrombotic microangiopathy (TMA). From the same center, another case report (Jhaveri et al. Kidney International 2020) described a COVID-19 patient’s TMA in more detail and presented the biopsy findings below.
Jhaveri et al. Kidney International 2020 (A) PAS. Diffuse coagulative cortical necrosis with widespread glomerular thrombi. (B) H&E. Glomerulus with microthrombi and coagulative necrosis of proximal tubules. (C) Silver stain. Thrombosed glomerulus. (D) EM. Extensive fibrin deposits in capillary lumens and partially denuded capillary.
Tubuloreticular inclusions, also known as “interferon footprints” that are prominent in HIV associated nephropathy, were found in some biopsies.
Gaillard et al. Kidney International 2020. (A) PAS. Collapsing of glomerular tuft, ATN. (B) Tubuloreticular inclusion (arrow)next to glomerular basement membrane (white asterisk).
Does SARS-CoV-2 infect the kidney directly?
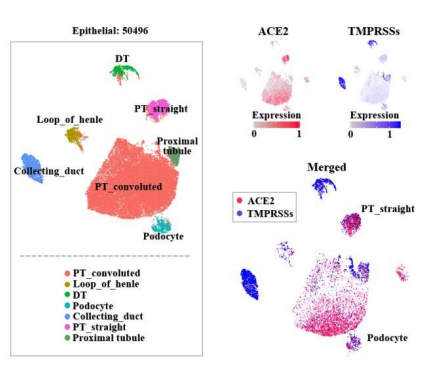
So how does SARS-CoV-2 infection contribute to kidney injury in the proximal tubule and, for some, the glomerulus? The present virus binds with ACE2, which is well expressed in the kidneys (particularly in podocytes and proximal tubular epithelial cells per single-cell RNA sequencing data reported in a preprint (Xu et al, Preprints, 2020, expression plot below), and could serve as a binding site and potential injury mechanisms.
One possibility to consider is that pre-existing AKI induced by severe sepsis may be exacerbated by SARS-CoV-2. Once the initial kidney injury occurs and alters the integrity of cell-cell junctions, the virus might then gain access to ACE2 on the luminal surface. ACE2 is expressed on the apical membrane, and it is unclear if the virus is capable of gaining entry to the luminal compartment.
Coronavirus entry into host target cells also requires fusion of the viral envelope with cellular membranes. Fusion-activate SARS-CoV peptides are created by specific proteolytic cleavage of the S proteins in a step called “priming.” As a consequence, cell infectivity not only depends on ACE2 expression but is also governed by types of proteases found in a given cell type. In the kidney, detectable levels of the TMPRSS2 (transcript mouse, Ransick et al. Dev Cell, 2019), which primes the SARS-CoV-2 S protein, are only detectable in the proximal tubule S3 segment. Even then, TMPRSS2 is expressed at very low levels. It remains to be determined if other TMPRSS or other proteases in the proximal tubule can mediate the priming of SARS-CoV-2.
Whether SARS-CoV-2 is present in kidney cells of patients with AKI remains unclear. Immunohistochemistry in the preprint (Diao et al, MedRXiv 2020) figure below demonstrated the presence of SARS-CoV-2 nucleocapsid (NP) protein in the kidneys, with the authors hypothesizing potential direct tubular injury from the virus.
Diao et al., medRXIV, 2020. This figure shows the immunohistochemistry staining pattern of SARS-CoV-2 NP in kidneys (A-C) and lung (D) of COVID-19 patients. Panel E reveals no SARS-CoV-2 NP antigen staining in normal kidney (E) and negative antibody control (F). The primary antibody used for IHC is clone ID: 019 rabbit IgG developed by Sino Biological in Beijing. Antibody dilution was 1:100.
Su et al. also reported the presence and detection of viral particles in the tubular epithelium and podocytes, as seen in the figure below.
However, Larsen et al. did not detect any virus RNA by RNAscope in their case report.
Larsen et al., Kidney International, 2020. RNAscope analysis for housekeeping gene (a, brown) and SARS-CoV-2 (b) reveals no signal for viral RNA in kidney parenchyma.
The issue has become a little bit more complicated as more biopsy series have been published. The correspondence pages of Kidney International created a buzz regarding whether the particles noted on EM are actually the virions (Miller et al, Kidney Int, 2020; Kissling et al. Kidney Int 2020), or clathrin coated pits or even multivesicular bodies (Calomeni et al, Kidney Int 2020; Roufosse et al. Kidney Int 2020, Pesaresi et al. Eur Rev Med Pharmacol Sci 2020). See some (deliberately unlabelled) pictures below for you to ponder on.
Images from Miller et al (top left), Su et al (top right), Calomeni et al (bottom left) and Walawalkar et al, Twitter (bottom right).
Lastly, this autopsy study from Germany (Puelles et al, NEJM, 2020) and this autopsy study from Michigan (Farkash et al, JASN 2020) did detect viral RNA in kidney tissue, while others did not (Couturier et al. Clinical Kidney Journal 2020, Kudose et al. JASN 2020, Golmai et al. JASN 2020, Lazareth et al. AJKD 2020, Sharma et al. JASN 2020, Rocha et al. Laboratory Investigation 2020, Wu et al. JASN 2020, Rossi et al. KI Reports 2020).
In summary, to paraphrase from the NephronPower blog
ATN is by far the most common presentation for AKI (if not prerenal), even in the transplanted kidney. Pigment nephropathy from myoglobin or hemoglobin is rare. Vitamin C overdose induced oxalate nephropathy is rare.
Podocytopathies are the most common glomerular findings
Other glomerular diseases are seen, but less common (TMA, ANCA, Membranous GN, anti GBM)
The virus was not found in the kidney with immunohistochemistry in all 3 studies.
Does the kidney get infected?- time will tell.. data is mixed
Is it possible for SARS-CoV-2 to be present in urine or Continuous Kidney Replacement Therapy (CKRT) or PD fluid effluent?
There are preprint reports that SARS- CoV-2 has been isolated from urine of patients in Guandong by Peng (medRxiv 2020) and in Wuhan by Wang (medRxiv 2020). The presence of viral RNA has not been established as a direct link to kidney injury. The largest published to date case series by Guan (NEJM 2020) also noted the presence of the virus in the urine of one patient (in the supplemental table S2; though it's not clear how many urine specimens were tested). However, Wang (JAMA 2020) was unable to find the virus in 72 urine specimens. On the other hand, virus shedding was most common from bronchoalveolar lavage (93%), followed by sputum (72%) and even feces (29%). Of interest to concerns about CKRT effluent: only 1% (3 of 307 samples) samples showed virus in the blood, so this lowers the likelihood that effluent would be infectious. Another paper from Germany reports on detailed assessment of different body fluids over time in 9 patients (Wölfel et al, Nature 2020). While they isolated it from many other fluids, it was never detected in either blood or urine. Absence in blood, suggests it would be less likely to make it to the PD fluid as well, but with the presence in gut, it would be difficult to comment on this issue with confidence.
Shedding of coronavirus (SARS) in the PD effluent was investigated in 2004 (Kwan et al, JASN 2004), in the 7 PD patients in this study SARS virus could not be detected by PCR or viral culture.
There is a paper describing the isolation of the virus in a patient from Pisa, Italy (Coccolini et al, Annals of Surgery, 2020)
Another report of the presence of the virus has been retracted by the authors (Foque et al, Bulletin de la Dialyse a Domicile, 2020)
A third case report also describes the virus presence (Gisella et al, Kidney Int, 2020) with a sensible editorial note at the end, which says
‘it will be of critical importance to go beyond PCR testing and actually attempt to culture the virus from the fluid. At this time it is not clear if a positive PCR for SARS CoV-2 means contagious virus is present’
According to the recent ISPD-ISN webinar, there are several samples being processed both in New York and in the UK. In the meantime, PD fluid should be disposed of according to the established standard of care - adding 500 mg/L chlorine-containing solution for 1 hour before disposing of the fluid in the toilet avoiding any splash. Stay tuned.
What about the urinary sediment?
Cheng et al (Kidney Int 2020) studied 701 patients at a single center. The incidence of proteinuria on admission was 44% and hematuria was 27%. Whether proteinuria and hematuria were due to AKI or underlying CKD is unclear as admission serum creatinine (SCr) values were called as baseline SCr; no pre-hospitalization SCr data were presented. Notably, only 5.1% of this study’s patients experienced a rise in SCr meeting KDIGO criteria for AKI during their hospital course. In a case series by Cau et al (medRxiv 2020) of 198 patients from Shanghai, they found 36% had proteinuria on admission. Another case series by the Anti-2019-nCoV Volunteers (medRxiv 2020) of 51 selected patients, proteinuria was present in 63%. This was mostly mild proteinuria (half the proteinuria being only 1+ on dipstick, and 2+ representing an additional third). In 64% of these patients, the proteinuria was seen on the first day of admission. Only 11 patients developed AKI in the form of elevated creatinine. Of the 27 patients who had a CT scan that included the kidneys, all of them had inflammation and edema of the kidney.
It is not clear what these numbers mean as probably do represent sicker patients who needed hospitalization. Additionally, proteinuria with febrile illness is common, as high as 74% of children and 83% of adults. Jensen (Acta Medica Scandinavica 1974).
Another report from Ochsner, New Orleans of 20 patients who underwent microscopic examination of the urinary sediment sheds more light on this topic (Hernandez-Arroyo et al, Kidney 360, 2020). Though proteinuria and hematuria was quite common in this case series of patients with AKI, these patients mostly had acute tubular injury. Additionally, in 6 patients in whom tubular injury was not clinically suspected, urinanalysis helped clinch the diagnosis, proving its ongoing utility. Interestingly, 3 patients of 20 underwent a kidney biopsy due to a clinical suspicion of glomerular disease, and had collapsing glomerulopathy.
Does AKI associate with worse outcomes?
Of course it does. We know this from the non-COVID-19 literature already. As an example, this multinational study by Hoste (Intensive Care Med 2015) of 1032 critically ill patients reported increased in-hospital mortality with all stages of AKI, with a hazard ratio (HR) increasing from 1.7 for AKI stage 1 to 6.9 for AKI stage 3. This is after adjusting for all possible covariates. Even for long term outcomes, AKI has been shown to more than double mortality risk compared to non-AKI survivors in this systematic review by Coca et al (AJKD 2009). Lastly, even the duration of AKI matters: longer duration of AKI is associated with worse longer term cardiovascular outcomes as well as risk of CKD. Mehta (BMC Nephrology 2018)
From the 2005 SARS experience, the mortality rate was much higher in patients who developed AKI: 92% versus 8% in those who did not develop AKI. Chu et al (Kidney Int 2005)
The first reports from the US are now coming out, showing
a much higher incidence of AKI than the reports from China and
a better sense on the mortality associated with AKI.
In a study from the Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium of 5,449 patients hospitalized with Covid-19 between March 1, and April 5, 2020, at 13 academic and community hospitals in metropolitan New York, the incidence of AKI was 36.6%, and 14.3% required acute RRT (Hirsch et al, Kidney Int, 2020). Approximately 90% with respiratory failure developed AKI, and half of the cases of AKI in those with respiratory failure met the criteria AKI within 24 hours of intubation. Mortality was 35% in those with AKI compared to 6% in those without AKI.
A preprint study from the Mount Sinai Coronavirus Informatics Center and the Mount Sinai Division of Nephrology demonstrated very similar findings from 3,235 patients from 5 centers in the Mount Sinai Health System in NYC (Chan et al, MedRxiv, 2020). AKI occurred in 46% of patients admitted with COVID-19, 20% of these required acute RRT, and 80% with respiratory failure developed AKI. Mortality was 41% in those with AKI and 7% in those without AKI. Notably, of those that developed AKI, only 30% survived and recovered kidney function. In survivors, 46% of patients still had persistent AKI (AKD) at the time of discharge.
These data are consistent with another recent report from New Orleans that reported an incidence of AKI 28%, along with high incidence of RRT (55%) and mortality (50%) in 575 patients admitted to the Oschner Center Medical Center ICUs with COVID-19 (Mohamed et al. Kidney360, 2020). Of the 155 with respiratory failure, 65% developed AKI. Adjudication of etiology of AKI suggested that 66% had ischemic AKI and of importance, only 9% were felt to be due to prerenal etiology.
Moreover, all three studies (Northwell, Sinai, Oschner) found that proteinuria (69-85%) and hematuria (64-75%) are extremely common in COVID-19.
The US-based studies demonstrate a much greater severity of AKI and associated mortality than the studies from China. More work will be need to discern why this is, but one reason may be the higher burden of comorbidities in the US population (Northwell, Sinai, Oschner) vs. China (eg Guan et al, NEJM 2020 and Cheng et al, Kidney Int 2020), as shown below.
The second reason may be due to differences in ACE2 expression between Asians and Occidental persons in various nephron segments. As stated in a recent perspective piece (Batlle et al, JASN 2020):
Interestingly, a recent study described single-cell transcriptome analysis in 15 normal human kidney samples. In this study, the proportions of kidney cells expressing ACE2, the SARS-CoV-2 binding site, and proteases of the TMPRSS family were compared between occidental and Asian individuals. Interestingly, the expression of ACE2 and kidney disease–related genes was higher in occidental donors relative to Asian donors. This would suggest that the susceptibility to kidney injury from coronavirus infection might be higher in individuals of occidental rather than Asian descent. We are not aware, however, of data supporting this possibility.
Fig S2B from Pan et al, Intensive Care Medicine, 2020
See the table below for a summary of the association of AKI with bad outcomes:
Sources: Cheng et al KI; Zhou et al, Lancet; Ruan et al, Int Care Med; Chen et al BMJ; Hirsch et al, Kidney Int; Chan et al, MedRXiv; ICNARC dataset (accessed May 8); Mohamed et al, Kidney360
The data from Cheng et al in KI, and Chen et al in BMJ probably represents the same dataset, see this rapid response in the BMJ. Similarly, we think the data from Zhou et al and Ruan et al are from the same dataset. We have excluded some studies from the previous version of the table (archived here) which only had patients who were dead or discharged, making the presented estimates unreliable.
How do we treat AKI in these patients?
The treatment of AKI seems to be no different than in other settings, but see more nuanced discussion below.
Should we strive to keep patients with COVID-19 and AKI wet or dry?
Since volume is an important and frequent reason to initiate KRT in this setting, sometimes even before traditional ‘uremic’ indications, close attention must be paid to avoiding over-aggressive volume resuscitation. At the same time, since these patients can also have high fever, they will have larger than usual insensible losses. Hence, one may also run the risk of unnecessarily causing pre-renal AKI. Hence, as always in medicine and nephrology, one should strive for euvolemia rather than a simple wet/dry heuristic.
Should we start KRT early for these patients?
In the non-COVID-19 literature, this question has been debated in depth. We know from the AKIKI trial (Gaudry NEJM 2016 and discussed on NephJC) and even in patients with AKI and sepsis, the IDEAL-ICU trial (Barber NEJM 2018 which made it to the top stories in 2018, #3 here) reported no difference in outcomes for early versus late starts. For the people who still hold out some hope, the STARRT-AKI trial is sure to put those doubts to rest (we hope).
Some people might say, ‘at least starting early is not harmful, so why not try?’ Well, in the AKIKI trial, about half the patients in the ‘delayed’ start arm never even required dialysis. In the setting of COVID-19, do we really want to expose healthcare workers to procedures that are not even needed in half the patients, and require additional manpower and machines? Until we know better, traditional clinical indicators for renal replacement therapy still rule in this setting.
Does the modality matter for treatment of AKI in this setting? Wouldn’t convection remove some bad cytokines?
Again, we look at the non-COVID-19 literature for this. For the critically ill patient, whether you perform continuous therapies (CVVH, or CVVHD, or CVVHDF) or hybrid therapies (PIRRT eg SLED, SLED-f, AVVH, AVVHD) there has been little reported that suggest there are differences in outcomes. The appeal of removing ‘bad humours’ may seem, well, appealing, but no good trial data supports this. Remember, convection doesn’t differentiate pro-inflammatory cytokines from anti-inflammatory cytokines. Use convection if that’s your standard practice, if not, diffusion should be good enough. If CKRT/PIRRT are not available, intermittent hemodialysis is acceptable.
A point that came up during the #NephJC discussion, was the possibility that consumables for CKRT (eg tubings and dialysate/replacement solution bags) may run low if usage is high and the supply chain becomes unreliable.
Does dose matter?
There is no evidence that higher dose of KRT improves outcomes, see the RENAL and ATN trials. Hence using the same dose that one usually uses for critically ill patients with AKI should be used for COVID-19 patients with AKI. If there are concerns about availability of consumables (dialysate/replacement fluids), consider using lower than usual rates after achieving metabolic control.
What is the role, if any, for hemoperfusion?
This editorial from Ronco and colleagues discusses the potential role of hemoperfusion, which can theoretically remove cytokines. However, the best quality data we have is from the EUPHRATES trial by Dellinger et al (JAMA 2018). This multicentre trial included 450 patients with septic shock and high circulating endotoxin levels. Polymyxin B hemoperfusion, which does remove endotoxins, was compared to sham hemoperfusion, and there was absolutely no difference in any outcome.
Is the risk of filter clotting higher in these patients? Why? And what can you do about it?
See ISTH (International Society for Thrombosis and Hemostasis) webpage with resources
Severe COVID-19 disease is accompanied by hyperinflammation, and inflammation in general resolves with a thrombotic process, so the thrombosis reported is not entirely unexpected. The subsequent fibrinolysis is reflected in the highly elevated D-dimers reported in COVID-19. Indeed, in critically ill patients with septic shock, the risk of venous thromboembolism is high, upto 20-30%. Previous data suggest use of heparin as thromboprophylactic dosing (Alhazzani et al, Crit Care Med 2013) reduces risk of pulmonary embolism without increasing bleeding risk, and this benefit may be even larger with low molecular weight heparins (LMWHs) which have a simpler dosing as well. So one should expect that most patients requiring KRT will be on prophylactic heparin or LMWH, though robust data on dosing (eg higher doses based on weight) or therapeutic dosing is still awaited.
Hence it is not surprising that greater filter clotting is also seen in these patients. The ASN webinar from Apr 30 (link to recording, slides available here, PDF) includes detailed advice on this topic including some discussed below.
Make sure you follow all the other ways of reducing filter clotting:
Check vascular access and access flow,
Central vein catheter requires immediate locking after insertion, consider locking with tPA if recurrent CVC thrombosis,
Higher blood flows,
Predilution replacement fluid administration for hemofiltration,
Dose heparin infusion appropriately. Some practical tips adapted from the KDIGO guidelines follow:
Consider using a heparin bolus, say 1000 units
Start pre-filter heparin at higher than usual rates [typically 500-700 units an hour (5-10 unit/kg/hr)] but anecdotally suggest starting higher at 1000 units/hour
Check a PTT before starting as 10-20% have hepatic dysfunction prior to starting CKRT
Check a PTT 2-4 hours after starting the heparin and target a 5 sec increase - as the goal is not to anticoagulate the patient but just the circuit. If your have not clotted after 2-4 hours you can either stand pat (if your pressures look okay on the machine) or consider increasing by 100-200 units/hr and rechecking PTT in 2-4 hours
Keep in liver injury as factors besides heparin can prolong the PTT
Check your filter; some filters may be less thrombogenic than others
Bivalirudin and Argatroban use if heparin induced thrombocytopenia (HIT)
Lastly, there is emerging anecdotal experience for the use of LMWH in this setting. Much of this will be off-label depending on your place of practice.
If one is not having clotting problems with the KRT circuits then this may not be needed. The protocol below is based on sound physiological principles, and experiences in treating COVID-19 patients, but there is limited evidence of its efficacy or safety as it has not been formally tested in a trial. The algorithm below has been provided from the Rush University Medical Centre.
What about using regional citrate anticoagulation?
If your centre has experience in using regional citrate anticoagulation, do so. If not, this is likely not the time or place to start learning how to do this. However, if you are not in a crisis situation, it may not be a bad idea to get up to speed to put some training and protocols in place. Citrate anticoagulation can be tricky for the neophyte and one may end up doing more harm than good (see Davenport and Tolwani, CKJ 2009).
As a quick refresher, regional citrate is simply a means of lower the ionized calcium concentration without lowering systemic calcium levels. Remember the coagulation cascade with calcium being a cofactor for all the factors/enzymes. You can do that commonly by infusing ACD (acid-citrate-dextrose) in the extracorporeal circuit and infusing calcium intravenously systemically. Then, you have to make sure your dialysate and replacement fluid is calcium free. Lastly, you have to monitor post-filter ionized calcium (to make sure extracorporeal circuit is anticoagulated) and systemic ionized calcium (to make sure patient is normocalcemic) and titrate infusions accordingly. Other options to ACD are trisodium citrate or a citrate containing replacement fluid (eg as described here or if available a commercial preparation such as Prismocitrate).
In view of severe clotting, some (but not all) programs have resorted to adding heparin (or LMWH) in addition regional citrate anticoagulation.
ECMO and CKRT: Some technical considerations
Hypoxemia is a major factor here, and some patients may end up on extracorporeal membrane oxygenation (ECMO) and need KRT. This review gives a good discussion of the technical issues, including the different options. The CKRT could be done in series, with the machine being typically connected to the venous limb of the roller-head ECMO circuit before the pump (see figure below). If a centrifugal pump is used, the CKRT machine should be placed after the pump, because of a risk of air entrapment if placed before the pump.
Figure from Askenazi et al, CJASN 2012
Another solution (especially if running low on CKRT machines) is by incorporating an in-line hemofilter into the ECMO circuit (see figure below). These hemofilters are designed for use with high pressure systems, and the fiber characteristics make diffusive clearance less effective than conventional membranes in this setting. In addition, the amount of ultrafiltration made is limited by the infusion pumps that maximize at approximately 1 L/h.
Figure from Askenazi et al, CJASN 2012
What can you do if there is a surge of COVID-19 and resources become limited?
How you respond depends on what is limited.
We are running out of CRKT machines
Convert from continuous to Prolonged Intermittent Kidney Replacement Therapy (PIKRT) For a review of PIKRT see Edrees et al (Adv Chronic Kidney Dis 2016) so you can use one machine for 10 hours on two patients a day, or three patients for 6 hours each. Consider running at higher flows (e.g. targeting 45-50 ml/kg/hour of effluent), if there is plenty of dialysate fluid stock.
Consider use of acute peritoneal dialysis (see below)
Use conventional HD machines and do SLED: decrease Qb to 200; Qd to 100 ml/min (or as low as the machine will go, some go only up to 300); consider using the smallest surface area filter you have (eg a pediatric filter with 0.6m^2 surface area) and run for 8 to 10 hours. This will need redeployment of HD nurses to ICU, which will be major concern.
Use conventional hemodialysis for IHD
We are running out of replacement fluid and/or dialysate
Lower the dose. Use an effluent rate of 10-15 ml/kg/hr rather than 25 ml/kg/hr, especially after the first few hours once metabolic control has been achieved. This is below the guidelines for CKRT dose. See some calculations here about how much clearance you can get at lower doses (Shafi, Twitter, 2020).
Mix your own dialysate for CKRT. There are a number of recipes. Here’s one from Vanderbilt (Burgner et al CJASN 2020):
1 L 0.9% NaCl and whatever KCl you need
1 L D5W with 150 mEq NaHCO3
1L 0.9% NaCl with 1 gram MgCl2 (this is grams MgCl2 not the hexahydrate)
1L 0.9% NaCl with 1 gram CaCl2
Mix in a 5 liter dialysate bag and serve at body temperature
Using regional citrate? Then you need calcium free dialysate/replacement solution. See here for a simple method to make these from Bruce Mueller
Another method of preparing CKRT fluid from a conventional dialysis machine is described in this tweet thread from Chirag Parikh’s team
Consider use of acute peritoneal dialysis (see below)
Use conventional hemodialysis
We are running out of capacity for conventional hemodialysis
For stable patients without a lot of metabolic abnormalities,
Consider switching to two days a week hemodialysis
Consider switching to 3 hours dialysis
How can you conserve manpower and PPE?
Wait for clinical indications before starting KRT. Consider using high dose diuretics and K-binding resins (off-label) to delay dialysis if necessary. Key aspect is to watch volume status and not aggressively volume resuscitate every patient with AKI
For suspected COVID-19 patients who would require KRT with 1:1 nursing and PPE, consider postponing dialysis till you get results back (eg using K-binding resins or high dose diuretics).
Try cohorting stable COVID-19 positive patients together in last shift with a higher nurse:patient ratio, rather than do all of them in individual rooms with 1:1 nursing.
What are the access considerations?
Consider an internal jugular catheter as some patients who may develop ARDS may receive prone-position ventilation. Also, if a patient is being proned for ventilation, consider their future dialysis need and consider inserting a dialysis catheter prior to proning them.
Consider locking the catheters immediately after insertion with citrate/heparin as per local protocols, as these patients may be hypercoagulable.
Strictly not about AKI, but if there is a chronic dialysis patient who has an AVF/AVG, conventional thinking is that they need a central line. One may want to avoid unnecessary procedures in the COVID-19 setting. Rifai et al (Hemodialysis Int 2018) elegantly describes how it is possible to do CKRT using an AVF/AVG.
What about using acute peritoneal dialysis (PD) for patients with AKI if supplies for hemodialysis and CKRT run low?
Useful links for this section:
King’s College Acute PD in ICU protocol. (PDF Link)
ISPD ISN Webinar on PD in COVID19 associated AKI (Link)
ISPD Position paper (PDF Link)
Renal Association adaptation of NICE guidelines (PDF Link)
Peritoneal Dialysis for Acute Kidney Injury Treatment in the United States - (Srivatana et al, Kidney360, 2020)
Peritoneal Dialysis During the Coronavirus Disease-2019 (COVID-19) Pandemic - (El Shamy et al, Kidney Medicine, 2020)
Acute PD seems like an ingenious solution in these times. The International Society for Peritoneal Dialysis has published guidelines for PD in AKI (Cullis et al, PDI 2013). New guidelines are being reviewed currently and are expected soon. A few details from the new guidelines were discussed in a recent ISN-ISPD webinar and are enumerated below.
Advantages
Relatively simple to insert bedside for ICU patients by IR/Surgeons or Interventional Nephrologists.
Biocompatible membrane and better cardiovascular stability
Avoid vascular access and need for anticoagulation
Better renal outcomes?
Disadvantages
Precise ultrafiltration is slightly more difficult to identify on a cycler (not very intuitive real-time reading) and difficult to prescribe (cannot dial in the required UF)
Increased abdominal pressure affecting ventilation?
May need innovative exit sites and dialysis scheduling to accommodate prone ventilation.
ICU team may not be very familiar with acute PD in the ICU setting.
Prescription
Several society papers and guidelines listed above have developed protocols for acute PD prescription in the ICU setting (links above). A few highlights -
Automated PD - high volume PD with hourly exchanges for the major part of the day or more.
Targeting a weekly kt/v urea of 3.5 on PD provides comparable outcomes to daily IHD (minimum dose 2.1 may be acceptable). (ISPD Guidelines)
PD kt/v calculation is simple
K = volume of dialyzate drained in 24 hours x D/P urea
T = duration of dialysis
V= Volume of distibution ~ 0.5 (F) or 0.6(M) x Body weight
Tidal PD with 25 litres with 70% tidal volume per 24 hours has similar outcomes to CVVHDF with the effluent of 23 ml/kg/hr
PD will never have very low urea, creatinine values as compared to CVVH - however, these do “high” values do not seem to affect the outcomes (attested by physicians doing acute PD for COVID19 in UK and US).
Prone ventilation - Several questions were raised about prone ventilation and PD. While there are case reports (Klisnick et al, PDI 1998) published previously, current experience is still unpublished and anecdotally the following has been tried to accommodate prone ventilation with PD
Lateral exit site - to access the PD catheter when prone
Emptying the abdomen/hold PD completely for the duration of prone ventilation
Pillow supports to the chest and pelvis during prone position to counter the pressure on the abdomen in prone position.
Monitoring the IAP and adjusting PD fluid volume and prescription based on IAP and ventilatory settings.
Overall Opinion on PD in AKI for COVID19
Feasible - can be considered for critically ill patients with some innovative measures.
Will certainly preserve CVVH resources
If option available - use PD for less hypercatabolic patients.
Mechanical complications were low overall as patients invariably supine throughout.
Increased workload on PD teams as ICU teams may not be initially comfortable with the modality.
Experience from UK (King’s College) and US (New Orleans) is encouraging.
Is there any data on acute PD from other scenarios?
Over the years several studies have demonstrated equivalent benefit in acute PD even in the ICU setting. Some publications were tabulated by the ISN-ISPD team to create the following tables presented during the seminar.
In a situation when PD fluids are not readily available, PD fluid can be reasonably prepared using the ISPD guidelines. An important caveat is to monitor electrolytes more carefully as the composition of these DIY solutions are different than the standard commercially available PD solutions.
Specifically, this might be a hypercatabolic state, can PD actually achieve metabolic control? This prospective study by Chitalia et al (Kidney Int 2002) in patients malaria and AKI reported adequate clearance with tidal PD, and similar results were reported by Ponce (CJASN 2012) when she looked at AKI in patients in Brazil. The latter study used flexible PD catheters, and also reported improvement in respiratory parameters - which is also a concern given the increase in abdominal pressure from abdominal peritoneal fluid.
What are considerations for drug dosing in AKI, especially when they need KRT?
There are no proven and established medicines which have been clearly shown to be beneficial in COVID-19. However, it is possible that they will be used in patients with AKI, so it is useful to review the clearance of these drugs. Some of them (e.g. chloroquine) have been around for many years, whereas others (eg remdesivir) have not been officially licensed/approved for open use yet, with access mostly from compassionate use or as part of clinical trials. For drugs such as chloroquine, many different dosage regimens have been floated around (mostly because of the woeful lack of evidence and leaps of faith involved), so a careful review of pharmacokinetics and discussion with a pharmacist is useful.
Apart from the molecular size (ie small molecules will be removed by dialysis), and protein binding (protein bound drugs are not removed by dialysis), the other major criterion is the volume of distribution. With a large volume of distribution (eg drug present in tissues, and not just in blood), even if dialysis removes it from the circulation, it won’t be effectively cleared, because of high levels in the tissues. Again, for chloroquine, this is quite high - which means most of it is distributed in the tissues, and dialysis will not remove a significant amount.
Names in italics refer to brand names. Vd: volume of distribution
1: This is a common reported exclusion. See discussion of this in this Twitter thread, and more detail below
2: Dose in gout is usually 0.6 mg, this dose is reported for the COLCORONA trial, which has CrCl < 30 as an exclusion
3: These drugs do have some clearance by kidneys, so accumulation with occur with chronic dosing, not relevant in the COVID-19 setting
Specifically, what should we know about Remdesivir and its dosing in AKI?
Remdesivir is a prodrug (or a proTIDE, aka pro-nucleotide to be precise) that gets converted to GS-441524 in vivo, which is the active anti-viral agent. Its phosphorylated form functions as an adenosine nucleotide triphosphate analog, which interferes with the action of viral RNA-dependent RNA polymerase, decreasing viral replication (Eastman et al, ACS Cent Sci 2020).
The prodrug half life is about 20 minutes after intravenous infusion, but the active metabolite has sustained intracellular levels, and a half life of about 20 hours (Sheahan et al, Sci Trans Med 2017). Most of the elimination is through the kidney (~75%) and 18% in feces. Not much is known about its volume of distribution or protein binding in the literature, but given its half-life and rapid intracellular entry, volume of distribution would be expected to be high.
The dosing in AKI is complicated less because of the drug, and more related to the excipient, a cyclodextrine (sulfobutylether-β-cyclodextrin sodium salt or SBECD). SBECD is cleared by the kidneys and accumulates when the GFR is < 30 in particular. There is additional concern that SBECD can cause AKI via osmotic tubulopathy, with anecdotal reports from its use as an excipient for voriconazole (F. Marty, Twitter, 2020). Hence once the GFR < 30, even the FDA fact sheet (link, PDF) suggests using risk/benefit analysis to guide its usage, with some advice from experts (F. Marty, Twitter, 2020) that the risk/benefit suggests we should use it on patients on KRT. Since based on the information we have with a molecular weight of 600 Da, it is likely to be cleared with convection, so dosing while on CKRT should take that into account. It would be reasonable to give the dose after HD in patients on hemodialysis. Also see this comprehensive review of the topic (Adamsick et al, JASN 2020) which suggests similar advice.
An additional wrinkle is a recently added warning on remdesivir and AKI. There does not seem to be any firm basis for this. There was no AKI signal in the preliminary ACTT results (Beigel et al, NEJM 2020), nor in the ACTT final results made available on clinicaltrials.gov, as seen below:
Indeed, the 10 day duration of remdesivir was associated with more AKI than the 5 day duration (see Goldman et al, NEJM 2020), at 8% with 10 day course versus 2% with the 5 day - which we might speculate could be where the excipient accumulation might be playing a role.
Any concerns with using statins for ARDS
HMG-CoA reductase inhibitors suppress a number of mechanisms that drive the development of ARDS. This has lead to investigations in the use of statins to ameliorate lung injury in ARDS. This strategy works in disease models of ARDS and sepsis. Terblanche (Lancet Inf Dis 2006) Jacobson et al (Am J Physiol Lung Cell Mol Physiol 2005), Two large multi-center randomized controlled trials have tried this in humans. Neither were able to show any benefit. McAuley et al (NEJM 2014) used 80 mg of simvastatin without benefit (or harm). The ARDS Clinical Trials Network (NEJM 2014) studied 20 mg of rosuvastatin and also found no benefit but a slight (but statistically significant) decrease in the time to renal failure (meaning more renal failure with rosuvastatin).
So, is AKI an independent risk factor for mortality?
We haven’t seen any good data to comment on this. eg in the paper from Zhou et al (Lancet 2020), 27/28 patients with AKI died. However, the comparisons are between 54 patients who died and 137 who were discharged. Data on 613 patients who were admitted was excluded from the study. In the most recent data from Cheng et al (Kidney Int 2020), AKI stage 2 or higher did persist in the multivariable model. These analyses are underpowered, but even if studies show "AKI independence", we know with 99% probability that most of it is still residual confounding which is simply the signal for identifying the sickest patients.
So, why make a big deal of AKI being an independent predictor of mortality?
Partly because we want to know that a functioning kidney matters. But what does it really mean? Steve Coca, who has thought deeply about this topic, shares his opinion below on the topic:
The AKI independence argument is a bit disingenuous. The other covariates (demographics, comorbidities, severity of illness score, baseline labs, PaO2:FiO2, whatever other variables) are usually only the baseline/admission variables. No other place do we give the organ the chance to "count" as we do the kidney. We look back and take the PEAK creatinine, out of the hundreds of creatinine values during an ICU stay, subtract the baseline from it, stage people, and then throw it in the model, and say "Aha! AKI is associated with every outcome short and long-term". The peak creatinine picked up every little bit of badness along the way- every dip in BP, every drop in O2 sat, every wave of endotoxin or viremia, every bleeding episode, and harnessed that into the one composite of kidney function/AKI.
List of Updates:
March 21: Page began
March 24: Link to ISPD g/l, Byrd’s words added; Contributors added
March 26: Added paragraph about Statins and ARDS
March 28: Table on drug dosing updated
March 29: Added suggestions for dealing with kidney replacement therapy shortages.
Apr 2: Added contributors; expanded on clotting and anticoagulation; expanded section on what to do when resources overwhelmed; added details on acute PD; CVC issues
Apr 6: Added contributors; added higher heparin dose need; Nature paper on absence of virus on urine, updated tables, caution about acute PD
Apr 12: Added contributors; Expanded section on histopathological findings from Su et al (Kidney Int) and Larsen et al (KI Reports)
April 14: Updated table on incidence of AKI, drug dosing; added links on making CKRT solutions in house
May 5: first AKI data from US added; cause of AKI clarified; sectionon clotting expanded with section on LMWH
May 7: Added links to UK Renal Association and NICE guidance + some details on remdesivir, nuanced volume section
May 15: Added data from 3 new US studies; acute PD information and protocols; controversy and correspondence about presence of virus in the kidney and the PD effluent
June 3: Added link to BMJ NICE guidance; new table on AKI and KRT need in the ICU; link to paper on urinanalysis findings
July 26: Extensive details of several papers with details of biopsies: Kudose et al. JASN 2020, Peleg et al. KI Reports 2020, Couturier et al. Clinical Kidney Journal 2020, Lazareth et al. AJKD 2020 Sharma et al. JASN 2020 Jhaveri et al. Kidney International 2020 Miller et al, Kidney Int, 2020; Kissling et al. Kidney Int 2020 Calomeni et al, Kidney Int 2020; Roufosse et al. Kidney Int 2020, Pesaresi et al. Eur Rev Med Pharmacol Sci 2020 Farkash et al, JASN 2020 Golmai et al. JASN 2020, Rocha et al. Laboratory Investigation 2020, Wu et al. JASN 2020, Rossi et al. KI Reports 2020
Oct 4: update on remdesivir