#NephJC Chat
Tuesday August 9th 9 pm EDT
Wednesday August 10th 9 pm IST
N Engl J Med 2022 Jun 30;386(26):2459-2470. doi: 10.1056/NEJMoa2202707. Epub 2022 Jun 17.
Restriction of Intravenous Fluid in ICU Patients with Septic Shock
Tine S Meyhoff, Peter B Hjortrup, Jørn Wetterslev, Praleene Sivapalan, Jon H Laake, Maria Cronhjort, Stephan M Jakob, Maurizio Cecconi, Marek Nalos, Marlies Ostermann, Manu Malbrain, Ville Pettilä, Morten H Møller, Maj-Brit N Kjær, Theis Lange, Christian Overgaard-Steensen, Björn A Brand, Marie Winther-Olesen, Jonathan O White, Lars Quist, Bo Westergaard, Andreas B Jonsson, Carl J S Hjortsø, Nick Meier, Thomas S Jensen, Janus Engstrøm, Lars Nebrich, Nina C Andersen-Ranberg, Jacob V Jensen, Neeliya A Joseph, Lone M Poulsen, Louise S Herløv, Christoffer G Sølling, Susan K Pedersen, Kurt K Knudsen, Therese S Straarup, Marianne L Vang, Helle Bundgaard, Bodil S Rasmussen, Søren R Aagaard, Thomas Hildebrandt, Lene Russell, Morten H Bestle, Martin Schønemann-Lund, Anne C Brøchner, Claes F Elvander, Søren K L Hoffmann, Michael L Rasmussen, Yvonne K Martin, Fredrik F Friberg, Herman Seter, Tayyba N Aslam, Sigrid Ådnøy, Philipp Seidel, Kristian Strand, Bror Johnstad, Eva Joelsson-Alm, Jens Christensen, Christian Ahlstedt, Carmen A Pfortmueller, Martin Siegemund, Massimiliano Greco, Jaroslav Raděj, Miroslav Kříž, Doug W Gould, Kathy M Rowan, Paul R Mouncey, Anders Perner, CLASSIC Trial Group
PMID: 35709019
Introduction
In patients with septic shock appropriate use of intravenous (IV) fluid resuscitation remains a major challenge for critical care medicine. Apart from resuscitation, they receive fluids for maintenance, as drug diluents, and as nutrition, and as a result they almost inevitably ending up with a positive fluid balance. This development of a positive fluid balance is associated with harm - including pulmonary edema, ARDS, AKI, and death (Boyd et al, Crit Care Med 2011; Malbrain et al, Anaesthesiol Intensive Ther 2014 ) - though confounding indications for IV fluid resuscitation makes drawing firm conclusions difficult. Sicker patients are more likely to receive a higher volume of fluids and hence more likely to have complications and die. On the other hand, lower volumes of IV fluids could also lead to under resuscitation and persistent shock. Initial resuscitation with 30 ml/kg within the first three hours of resuscitation, as per surviving sepsis guideline, is recommended (Evans et al, Intensive Care med 2021), however, this is based on low quality evidence - mostly observational studies, and the few trials being small and not definitive (Meyhoff et al, Chest 2020). Beyond the initial resuscitation, fluids are also given as part of ongoing resuscitation if required - and it is this phase the current CLASSIC trial looks to address.
Graphic from the RebelEM summary
Hjortrup et al published the results of their pilot CLASSIC study in 2016. This feasibility study showed that the protocol was able to achieve separation in IV fluid volumes (~ 1.4 liters mean difference during the ICU stay) delivered to patients in intensive care units (ICU) with septic shock. Though with only 151 study participants it was not powered to show a difference in outcomes, there were some signals that ischemic events and acute kidney injury (AKI) were reduced in the restricted fluid group. The same trial group performed a meta-analysis (Meyhoff et al, 2020) on the question, which included 637 patients from 9 randomized controlled trials (RCT), the largest of which was their pilot trial. They did not identify a mortality benefit for restricted IV fluid volumes, but acknowledged that the quality of available evidence was low. This led to this large, well-designed, RCT.
The study
Design
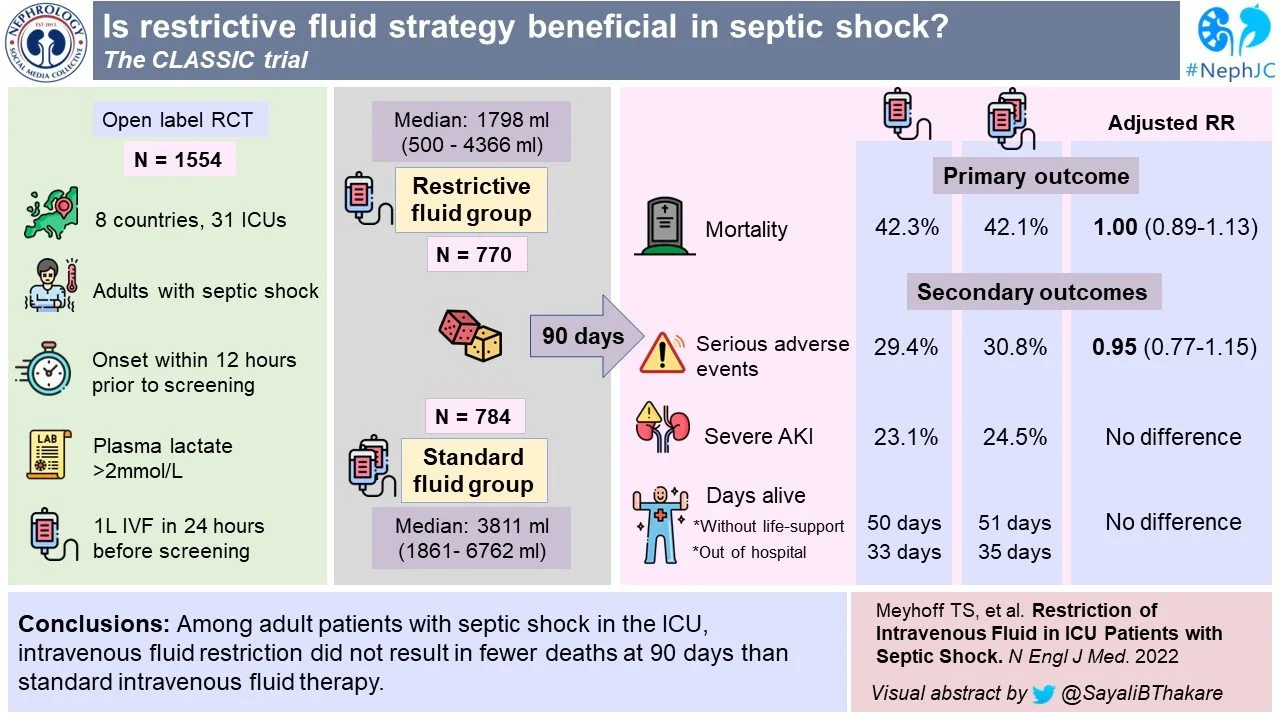
The Conservative vs Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care (CLASSIC) trial was an international, stratified, parallel group, open label, randomized-controlled trial. Eligible patients were randomly assigned in 1:1 ratio using computer generated allocation sequence. Therapy group assignments were open to patients, clinicians and investigators but masked from trial statisticians, as well as the data and safety monitoring committee. The stratification was based on trial site and presence of hematologic/metastatic cancer.
Inclusion criteria
Age ≥ 18 yr with septic shock -- defined according to Sepsis-3 criteria
Suspected or confirmed infection AND
Lactate > 2 mmol/L within the last 3 hrs AND
Ongoing circulatory support with vasopressor/ inotrope agent to maintain MAP of 65 mmHg or above AND
Received at least 1 liter of IV fluids in 24 hrs before screening AND
Onset of shock within 12 hrs of screening
Exclusion criteria
Life threatening bleeding
Acute burn injury involving > 10% of body surface area
Pregnancy
Consent not obtainable
Intervention
In the restrictive-fluid group, patients received IV fluids only when certain conditions were fulfilled:
When they had hypoperfusion defined by:
Lactate level of at least 4 mmol/L
MAP < 50 mmHg despite vasopressor or inotrope agent administration
Urine output < 0.1 ml/kg/hr in first 2 hrs after randomization
Mottling beyond the kneecap (mottling score > 2)
To replace documented fluid losses (e.g., gastrointestinal or drain losses)
To correct dehydration or electrolyte deficiency because the enteral route was contraindicated
To ensure a total daily fluid intake of 1 liter, including fluids with medication and nutrition, when the enteral route was contraindicated
If patients met any of the above criteria, a bolus of 250 to 500 ml of isotonic crystalloid was given.
For the standard fluid group, IV fluids could be administered with no set upper limit, and under the following conditions:
IV fluids could be given as long as the patient had improvement in hemodynamic factors, as described in the 2016 Surviving Sepsis Campaign guidelines.
To replace expected or observed losses to correct dehydration or electrolyte derangements.
As maintenance fluid, if the ICU had a protocol that recommended it.
No upper limit was set for the amount of intravenous fluids that patients in the standard fluid group could receive.
Enteral and oral fluids, nutrition (enteral or parenteral), and fluid used as a medium for the administration of medication were allowed in both groups.
The protocol included recommendations for types of IV fluids to be administered to patients in both intervention groups - isotonic crystalloids for circulatory impairment and losses, and albumin only if large amounts of ascites were removed by means of paracentesis. Concomitant interventions for septic shock i.e., relevant antibiotic agents and source control, vasopressor support, and renal replacement therapy (RRT) were administered as needed. All patients received the assigned intervention from the time of randomization until they were discharged from the ICU, for a maximum of 90 days. If a patient was readmitted within 90 days to an ICU participating in the trial, the assigned intravenous fluid intervention was resumed.
All other interventions, including the use of diuretics, were at the discretion of the clinicians.
Outcomes
Primary outcome
Death within 90 days of randomization.
Secondary outcomes
Serious adverse effects while in ICU, including cerebral, cardiac, intestinal or limb ischemia events, or a new episode of severe kidney injury.
Number of days alive without life support at 90 days.
Number of days alive and out of hospital at 90 days.
Sample Size and Analytic plan
For sample size, the authors estimated that 1554 patients would be needed - assuming a 45% 90 day mortality in the control group. This sample would allow detection of a 15% relative risk reduction (or roughly 7% absolute difference) with a two-sided alpha of 0.05.
Binary logistic regression analysis was used to compare data between both groups, adjusted for the starification sites (trial site and presence/absence of hematologic/metastatic cancer), as well as for the Simplified Mortality Score for ICUs, focus of infection, and use of systemic steroids. There was a per-protocol and prespecified subgroup analyses (as reported below). There were interim analyses when 105 and 30% patients were enrolled - for protocol adherence, and another interim analysis for safety when 50% (N = 777) had 90-day follow up data.
Funding
The study was funded by Novo Nordisk who had no role in design/conduct/ analysis/reporting of the results.
Results
The trial enrolled patients between Nov 27, 2018 and Nov 16, 2021 in 31 ICUs in the United Kingdom, Denmark, Norway, Belgium, Sweden, Switzerland, Czech Republic, and Italy.
Figure 1. Assessment, Randomization and follow up.
Of 2223 pts screened for eligibility, 669 were excluded (mostly for longer duration since sepsis diagnosis, followed by no provision for consent) and 1554 were enrolled, as planned.
Participants were 60% male, with a median age of 70 years. The median time from ICU admission to enrollment was 3 hours, and the median fluid volume received in the 24-hours prior to randomization was around 3 liters. About half of patients were on respiratory support. It is interesting to note that GI infections were the most common source of infection. See Table 1.
Table 1: Baseline characteristics of study participants
Fluid Administration
Median ICU stay for both groups was 5 days, and median time from hospital admission to randomization was 1 day. Over these 5 days, the restrictive fluid group received a median of 1450 ml, and the standard fluid group received a median of 3077 ml, excluding fluids given with medications and nutrition (Table 2). Though this is a difference of 1627 ml in fluids given, it’s worth noting that the day 5 difference in cumulative fluid balance was only 744 ml. Some of this was due to protocol violations - 162 (21.5%) in restrictive group and 101 (13%) in the standard group, and this was dealt with with a prespecified per protocol analysis.
Table 2: Fluid volumes and balances in two intervention groups
Outcomes
Death at 90 days occurred in 42.3% of patients in the restrictive fluid group and 42.1% in the standard fluid group (Figure 2A and Table 3). Thus the adjusted absolute difference in mortality was 0.1 (95% CI -4.7 to 4.9, p = 0.96) in the intention to treat analysis. The per protocol analysis also showed no difference (mean difference 0.6, 95% CI -4.9 to 6.1, p = 0.84).
Figure 2A: Survival curves censored at day 90 for the two intervention groups in intention-to-treat population
There was also no difference between groups on the number of days without single organ life support (whether pressors, RRT, or respiratory support), or days alive and out of hospital at 90 days after randomization.
Table 3: Primary and secondary outcomes
Subgroup Analysis
Subgroup analysis is shown in five pre-specified groups in Figure 2B. Unlike in the secondary outcomes when severe AKI was defined as KDIGO stage 3, here AKI was defined as KDIGO Stage 2 or 3. The plasma lactate subgroups were delineated based on samples taken within 3 hours of randomization. The eyes are drawn to the results for the respiratory support subgroups, which has a P-value of 0.03 for heterogeneity. Also note that about half the patients had already received ~ > 30 ml/kg fluid resuscitation before randomization.
Figure 2B: Primary outcome of death at 90 days in the five pre-specified sub-groups
Adverse events
29.4% in the restrictive fluid group and 30.8% in the standard fluid group experienced serious adverse effects. There were no differences between groups in rates of severe hypernatremia or hyperchloremic metabolic acidosis.
Discussion
The authors conclude that in this trial, they observed no significant differences in 90-day mortality or serious adverse events among the patients who received restricted fluid therapy compared to those who received standard therapy.
Strengths
The authors are congratulated on running the largest RCT looking at a difficult but important question. Very few participants were lost to follow-up, and tracking of fluid balance appears to have been meticulous. The difference in volume of fluids administered between the two groups was about only 2 liters at 90 days (median 1798 ml vs 3811 ml) but the cumulative fluid balance was separated by less than 1 liter (1645ml vs 2368ml).
The patient samples represented both university and community ICUs and most of the screened patients were in the trial - increasing generalizability.
Limitations
The separation between groups for cumulative fluid balance was not that huge: Higher rates of protocol violations in the restrictive group partially explain the modest achieved separation between groups. However, an additional reason is also apparent: many of the authors in this trial had been involved in the 2016 pilot trial with encouraging results, so it is possible their clinical practice (and those of other centers interested in enrolling patients in a fluid restriction trial) had already changed toward restrictive fluid use. Add in the Hawthorne effect that investigators participating in an open label trial of measuring and calibrating volume resuscitation may have behaved in a more conservative manner in this regard. McIntyre and Marshall, in their NEJM editorial, observed that the standard care arm received a lower volume of IV fluid compared to previous trials examining early, goal-directed therapy for septic shock.
These patients had already received a median of about 3 liters of fluid resuscitation in the few hours from presentation to randomization. As mentioned above, this trial does not answer the question of what should be the volume of initial fluid resuscitation. Indeed, ~ 50% patients had already received > 30 ml/kg fluids before randomization.
In the subgroup analysis, the group already requiring respiratory support seems to do a bit better with restrictive strategy. However this trial may have been underpowered to show a clear difference for this population.
In terms of generalizability, one should be careful not to extrapolate this as showing restrictive strategy as not being beneficial. In the current era of cautious and careful fluid resuscitation and early pressor support use, it may just have been difficult to demonstrate a large benefit of a restricted fluid strategy. How the restricted group would have compared to a different control group getting different ‘standard care’, i.e. a higher volume of resuscitation, is open for speculation. Additionally, the high proportion of GI infection as a cause of septic shock compared to pulmonary or urinary infections, may have increased the need for larger volumes of fluids, contributing to the negative results. One thought on this was as the study was done during COVID-19, with interventions to prevent airborne transmission using masks may have led to less pulmonary infections, as prior studies have had this the largest infective source.
Conclusions
In adult ICU patients with septic shock, IV fluid restriction did not result in fewer deaths at 90-days compared to standard IV fluid therapy. The signals for reduced AKI and ischemic events with restricted fluids in the CLASSIC pilot trial were absent in this much larger evaluation, though some may now generate a hypothesis that they are advantageous in those needing respiratory support based on the sub-group analysis, or it was harmful in those without need for respiratory support.
Despite the limitations, this was an excellent study which showed that the restrictive fluids strategy is safe, though not superior under these conditions.