#NephJC Chat
Tuesday May 10th 2022 9 pm Eastern
Wednesday May 11th 2022 9 pm IST 4:30 pm BST
J Am Soc Nephrol 2022 May;33(5):985-995
doi: 10.1681/ASN.2021060774. Epub 2022 Feb 23.
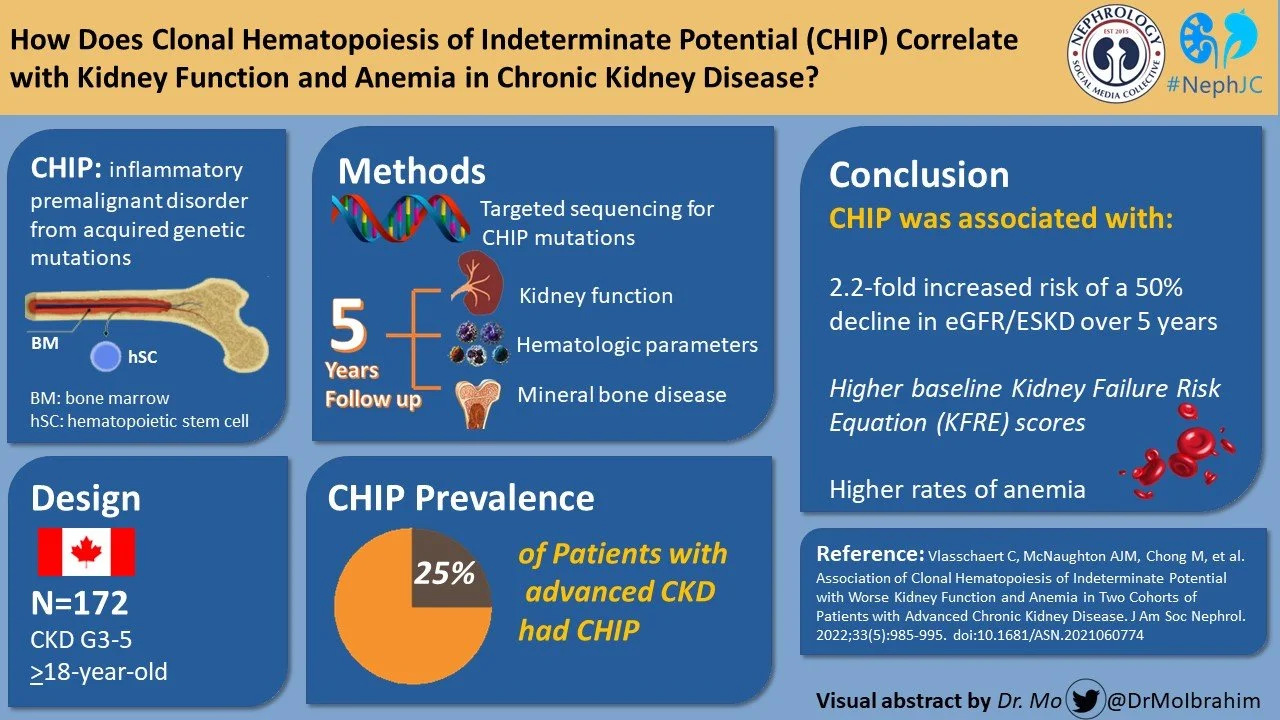
Association of Clonal Hematopoiesis of Indeterminate Potential with Worse Kidney Function and Anemia in Two Cohorts of Patients with Advanced Chronic Kidney Disease
Caitlyn Vlasschaert , Amy J M McNaughton , Michael Chong , Elina K Cook , Wilma Hopman , Bryan Kestenbaum , Cassianne Robinson-Cohen , Jocelyn Garland , Sarah M Moran , Guillaume Paré , Catherine M Clase , Mila Tang , Adeera Levin , Rachel Holden , Michael J Rauh , Matthew B Lanktree
PMID: 35197325
Introduction
Clonal hematopoiesis of indeterminate potential (CHIP) has hit the headlines recently as its presence confers a two-fold increased risk of coronary vascular events and 2.5 fold increased risk of ischemic stroke, the pathogenesis of which is largely driven by inflammation. Hence It is now understood that CHIP is a pro-inflammatory condition. Numerous population-based studies have reported that inflammatory marker levels are higher in chronic kidney disease. Could CHIP be the mediator of inflammation in CKD as well, and if so, what would be its implications?
Clonal hematopoiesis is the acquisition of genetically distinct acquired mutation of the subpopulation of myeloid cells. Clonal hematopoiesis arises as a result of endogenous mutagenic processes involving impaired DNA repair and altered dynamics of telomeres. The term ‘Clonal hematopoiesis of indeterminate potential’ (CHIP) was first used by Steensma et al in 2015. Clonal hematopoiesis of indeterminate potential is defined as clonal molecular genetic or cytogenetic changes in blood or bone marrow cells without signs of hematological neoplasm and absence of cytopenia. CHIP is a premalignant condition with a transformation rate of 0.5-1% per year. CHIP is distinct from monoclonal gammopathy of uncertain significance (MGUS), though it could be considered somewhat analogous in the clinical consequences, as discussed in further detail below. The characteristics of CHIP are summarized in the figure below.
CHIP Criteria Steensma et al 2015 Jul 2;126(1):9-16
CHIP promotes inflammation in the body and altered immune function which has been associated with an increased risk of multisystem morbidity and mortality (Jaiswal, Blood 2020). The figure below depicts how CHIP can cause aggravated inflammation.
Clonal hematopoiesis and inflammation, Elina K et al Experimental hematology, 2020
Like MGUS, CHIP is a pre-neoplastic state. But how are they different?. Predominantly the mutations of lymphoid progenitor cells lead to monoclonal gammopathy of unclear significance (MGUS) and mutations of myeloid progenitor cells lead to Clonal hematopoiesis of indeterminate potential. How MGUS and CHIP develop are represented in the infographic below.
CHIP is associated with an increased risk for transformation to myelodysplastic syndromes or acute myeloid leukemia. Rates of progression appear to vary with the specific mutations, the number of mutations, and the size of the hematopoietic clone. If clonal haematopoiesis is also accompanied by cytopenia, this is referred to as CCUS (clonal cytopenia of indeterminate significance). CHIP and CCUS differ from ICUS (idiopathic cytopenia of indeterminate significance) and IDUS (idiopathic dysplasia of indeterminate significance) by the evidence of clonality.
The table below summarizes variants of clonal hematopoiesis.
Variants of clonal hematopoiesis Steensma et al 2015 J
Moreover, CHIP is also associated with an increased risk of cardiovascular disease. In a study that included 17000 participants concluded that there was a two-fold increased risk of coronary vascular events and 2.5 fold increased risk of ischemic stroke (Jaiswal S NEJM 2017). This increase in cardiovascular risk might be more consequential than malignant transformation (seen in < 1% per year). The mechanism of increased cardiovascular events appears to be inflammation in the endothelium driven by clonally-derived monocytes/ macrophages as observed in a review (Libby P et al, JACC 2019).
The incidence of CHIP increases with age. Approximately 10-20% of persons over 65 yrs have CHIP. The various diagnostics available to detect CHIP are cytomorphology, FISH (Fluorescence in situ hybridization), and molecular genetics. CHIP is diagnosed in an individual if the percentage of mutant alleles reaches 2% in the blood, which equates to 4% of nucleated cells due to the heterozygous nature of the mutations found in CHIP
In CKD, the presence of CHIP is associated with lower cystatin-based eGFR (Dawoud et al, Leukemia 2022). What we don’t know is if CHIP is causative for adverse outcomes in patients with CKD, or if there was an association of CHIP with the progression to ESKD.
The Kidney failure risk equation (KFRE) is a useful tool in estimating the renal function decline over 2 and 5-year points in time. There are two KFRE: the 4 variable and 8 variable KFREs.The variables in KFRE include age, gender, eGFR, UACR, serum bicarbonate, serum albumin, serum phosphorus, and serum calcium. It is presented as % decline over 2 and 5 years. The 4 variable KFRE was used in the present study.
The present longitudinal cohort study analyzed the association of CHIP with the decline of kidney function and its complications in two independent Canadian populations with advanced CKD. The study was conducted to explore the hypothesis that adding CHIP to the KFRE could help with superior estimation of renal functional decline.
The Study
Study design
This study included data from two independent Canadian CKD cohorts. The first cohort is from the Kingston Health Sciences Center in Kingston, Ontario, Canada and the second cohort was from Canadian study of Prediction of Death, Dialysis, and Interim Cardiovascular evenTs (CanPREDDICT) which is an observational study from 25 sites across Canada. The inception of both CanPREDDICT cohort and Kingston cohort was 2009, and included adults with CKD with minor differences.
Inclusion, exclusion criteria and primary outcomes are tabulated in the figure below.
Chip genotyping and laboratory parameters included in the study are tabulated in the figure below.
An average of four measurements were taken for serum creatinine per patient per year in the Kingston cohort. Serum creatinine was measured by the IDMS method. Baseline eGFR was calculated using the CKD-EPI 2021 equation. For the CanPREDDICT cohort, the serum creatinine and other outcome data were measured at fixed intervals during a period of five years i.e at baseline, 0, 5, 1, 1.5, 2, 2.5, 3, 4, and 5 years. For eGFR-related outcome analyses, the Kingston cohort data were censored at 5 years to match the CanPREDDICT follow-up period. The kidney failure risk equation (KFRE) at 2 and 5 years was calculated using the baseline eGFR, UACR, age, and gender.
Statistical Analysis
The baseline characteristics of the samples were arranged according to whether CHIP was noted to be present or absent. For each CHIP variant identified, the variant allele fraction (VAF) was tabulated. The threshold of 0.02 VAF was arbitrarily derived, reflecting the technical limitations of the standard next-generation sequencing (NGS) and not the biological risk of leukemic transformation with lower frequency mutations.
In order to test for the association between baseline laboratory data with CHIP status and quantitative VAF, linear regression in univariable and multivariable analyses adjusted for age, sex, and baseline eGFR were used. Adjusted linear regression analyses were also used to assess baseline predicted risk of kidney failure, which was quantified by the 2- and 5- year KFRE equations.
In order to analyze the progression of kidney disease, participants were followed from the confirmation of CHIP status until the time that the primary outcome occurred. If necessary, they were censored due to death, loss to follow-up, or the end of the study period, whichever came first. Because there needed to be a way to estimate the association of baseline CHIP status with the time to kidney disease outcome, with adjustment for baseline age, sex, and baseline eGFR, Cox proportional-hazard regression models were constructed.
To assess the improvement of CHIP on 2- and 5- year KFRE predictions of ESKD, there were assessments made of nested Cox proportional-hazards models for evaluation for the best in fit. Examination for reductions in Akaike information criterion (AIC) and ANOVA-based likelihood testing were used in the assessment of model improvement via the addition of variables.
For quantification of the association between CHIP status and the slope of eGFR decline, linear mixed model regression was used. Participants with baseline eGFR>15 ml/min per 1.73 m2 were included in this analysis, and the observation period was limited to the first three years after CHIP genotyping to limit survival bias.
Results
A total of 181 participants from both the cohorts who met the inclusion criteria were included in the study. Samples of randomly selected 94 patients from the CanPREDDICT cohort were included, but 9 samples with > 50 putative somatic mutations were omitted due to suggested issues with library quality, leaving 85 patients enrolled in the study from this cohort.
The baseline characteristics of the study population are summarized in Table 1. Hypertension was present in almost 100% of the study population of both cohorts. Of note, the mean baseline eGFR was already quite low in the 20s, and lower in the CHIP group for both cohorts. Additionally, they were also older than those with no CHIP.
Table 1. Baseline characteristics of the two cohorts
Prevalence of CHIP in the Advanced CKD Cohort at Baseline
CHIP variants at a VAF >2% were found in 25% of the study population. DNMT3A was mutated in 39%, TET2 mutated in 37% and multiple genes were mutated in 27% of the cohort population. The largest detected clone was a VAF of 10% in the Kingston cohort and 15% in CanPREDDICT.
Participants with CHIP were older with lower eGFR at baseline, but the eGFR was not significantly low compared with patients without CHIP after adjusting for age and gender (-3.2土1.9 ml/min per 1.73 m2, P=0.08), which is not surprising given the small numbers.
Renal function decline as predicted by 2 and 5 year KFRE was significantly more in individuals with CHIP variants (2-year KFRE: 12.9土2.3% vs 8.8土0.8%, P=0.007; 5-year KFRE: 29.1土4.4% vs 19.9土1.8%, P=0.02) and the authors state that the difference persisted after adjusting for differences in age and gender. (see Figure 2A). That is unsurprising given that they had a lower GFR (statistical non-significance notwithstanding).
Figure 2A. Average baseline eGFR in individuals with and without CHIP variants.
Association of CHIP with Kidney Disease Progression
The primary outcome, development of ESKD or 50% decline in eGFR, was observed in 38.9% of the cohort during the follow up period of 5 years. The incidence rate of primary outcome was 0.196 per year in patients with CHIP and 0.113 per year in patients without CHIP variants. After adjusting for age, gender and baseline eGFR, the presence of CHIP was associated with 2.2 greater risk of 50% decline in eGFR or development of ESKD as depicted in figure 2C. Note that the patients start off with a mean GFR in the low 20s (with standard deviation ~ 10), and ESKD was defined as GFR of < 15 - so some participants either had ESKD at baseline (see vertical drop in blue line below) or reached that quickly enough. Unfortunately, the numbers at risk at each time point are not provided below the survival curve to help us understand this clearly.
Figure 2C. Survival curves for the primary composite outcome in individuals with and without CHIP
Perhaps a more important outcome was the change in GFR. Though the baseline eGFR was low in patients with CHIP variants, the eGFR decline was no different from patients with non CHIP variants in the first three years.(Supplemental figure 2A)
Supplemental Figure 2A Mixed model regression of eGFR trends by CHIP status for the first three years of follow-up
Adding CHIP to the KFRE after adjusting for age, gender and baseline eGFR, numerically improved the prediction of likelihood of decline in renal function similar to the 2 year and 5 year KFRE prediction model. (Figure 2D)
Figure 2D Iterative improvement in time-to-ESKD risk prediction by 2 year KFRE
CHIP and Complications of CKD
Baseline PTH (21.9土3.6 versus 13.0土1.0 pg/ml) and serum phosphorus(1.29土0.04 vs 1.19土0.02 mmol/L) were higher in participants with CHIP than in participants without CHIP. Serum bicarbonate(24.7土0.4 vs 26.2土0.3 mEq/L) and hemoglobin levels (11.8土0.2 vs 12.9土0.1 g/dl, P<0.001) were lower in patients with CHIP. Presence of CHIP correlated with high PTH and low hemoglobin but not with higher serum phosphorus and low serum bicarbonate after adjusting for age, gender and baseline eGFR.
MCV (94.3土1.4 vs 91.6土0.5 fl, P=0.03) and ferritin (269土51 vs 133土14 mg/L) are higher in patients with CHIP in comparison to patients without CHIP. There was no difference in transferrin saturation in participants with and without CHIP. This finding implies that probably the main driver for anemia in chronic kidney is not iron deficiency alone but contributed majorly by inflammation which is confirmed by increased ferritin levels which in turn are negatively associated with hemoglobin levels.
These associations between CHIP and complications of CKD are summarized in table 2.
Table 2: Association of CHIP and CKD complications
Discussion
People with CHIP are older and start off with a low GFR. To no one’s surprise, they develop a GFR < 15 more often (and are close to 15, to begin with). It seems to show a small change in the AIC in addition to the KFRE. Of interest, there were some associations with other CKD metabolic parameters such as anemia, and these patients had a higher ferritin as well.
The present study findings are in concordance with Cook and Dawoud who concluded that the prevalence of CHIP variants is higher in individuals with lower eGFR. The findings of low eGFR and progression of chronic kidney disease were also reported in a recent analysis by UK biobank which studied CHIP and association with adverse outcomes in chronic kidney disease.
CHIP mutations are found in 25% of advanced CKD patients at VAF>2%, the prevalence was similar to the Guermouche study. Detection of CHIP variants depends on various factors. Age, smoking, chemotherapy, active vasculitis, and sequencing methodology are some of the factors that influence the detection of CHIP mutations. CHIP mutations affect the hematopoietic stem cells. The effect on erythrocytes might result in lower hemoglobin levels, although anemia is not seen in the healthy population with CHIP variants. Anemia in CKD is multifactorial, one of which is chronic inflammation with IL-6 and hepcidin causing impaired iron absorption. How CHIP figures in all this remain to be seen.
Though the present study adds to the example role of CHIP in nonmalignant diseases, there remain many unanswered questions. First, how does CHIP contribute to the pathogenesis of different types of nephropathy? Second, what is the mechanism, if any, of mutant hematopoietic cells and kidney damage? Are the interstitial macrophages the culprit? Third, how does the CHIP influence the outcomes in chronic kidney disease?
The major limitation of the study was a small, homogenous sample population (almost entirely white) with fairly advanced kidney function. These findings need replication in a larger, longitudinal cohort. The patients in this study had fairly advanced CKD, and those with CHIP started off with lower GFR so that they were more likely to develop ESKD (i.e. GFR < 15) is not surprising. Possibly the more important result is the slope of GFR which was not different, CHIP or no CHIP. There appears to be only a marginal advancement of prognostication of renal function decline by adding CHIP to the existing KFRE. There was no mention of adding CHIP to the 8 variable KFRE estimation. Is 8 variable KFRE alone good enough to predict the renal function decline rather than adding CHIP remains unanswered.
Conclusion
CHIP seems like an interesting and possibly promising development. If the replication in larger cohorts holds up, future research aiming at animal models and studying chronic kidney disease histology in those with and without CHIP would help understand the mechanism of tissue damage by the mutant immune cells.
Summary prepared by:
Priyadarshini John, Nephrologist
Hyderabad, India
Christel Wekon-Kemeni, Resident Physician
North Carolina, United States of America
NSMC Interns, Class of 2022
Reviewed by:
Brian Rifkin, Jamie Willows, Swapnil Hiremath