#NephJC Chat
Tuesday July12th, 2022 at 9 pm Eastern and July 13th 11am AEST
Wednesday July 13th, 2022, at 9 pm IST and 3:30 pm GMT
Kidney Int 2022 Jun 22;S0085-2538(22)00461-6.
doi: 10.1016/j.kint.2022.05.024. Online ahead of print.
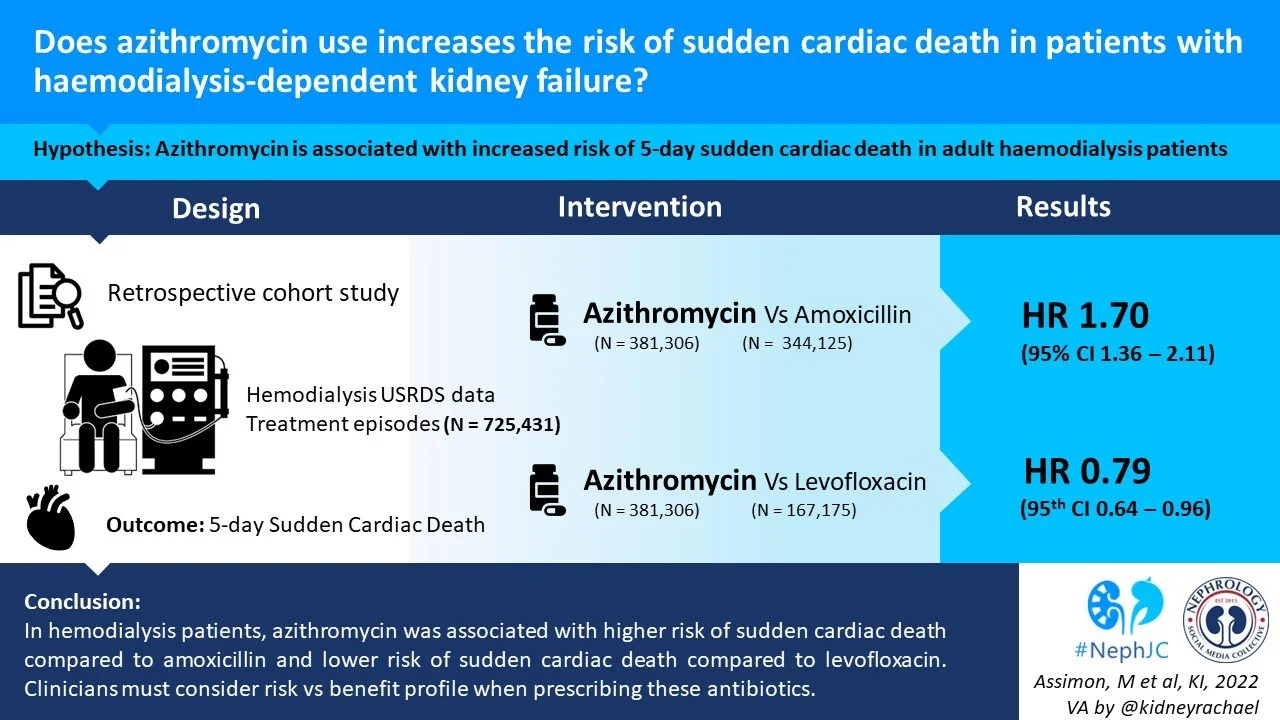
Azithromycin use increases the risk of sudden cardiac death in patients with hemodialysis-dependent kidney failure
Magdalene M Assimon, Patrick H Pun, Lily Wang, Sana M Al-Khatib, M Alan Brookhart, David J Weber, Wolfgang C Winkelmayer, Jennifer E Flythe
PMID: 35752324
Introduction
The rate of sudden cardiac death (SCD) is reported to be 20 times higher (Makar et al, AJKD 2017) in those on haemodialysis as compared to the general population. There are likely multiple reasons for this: increased incidence of coronary artery disease, structural cardiac pathology (including being more prone to arrhythmic triggers due to preexisting conduction problems), and/or electrolyte imbalance and fluid shifts that are unique to this population.
Commonly used antibiotics such as azithromycin and levofloxacin have pro-arrhythmic effects but nephrologists usually cannot quantify the risk of adverse side effects for their patients due to a lack of evidence comparing the cardiac safety of these antibiotics relative to others in patients on haemodialysis.
Azithromycin is a macrolide antibiotic, commonly prescribed for respiratory infections worldwide. In 2012, Ray et al reported concerns with potential adverse cardiac risks in those who took azithromycin. Since then, more data has emerged from multiple observational studies about its cardiovascular risk profile. Of the studies published, Rao, Chou, Mortensen and Zaroff et al reported an increased risk of adverse cardiovascular outcomes when compared to use of other antibiotics, but Svanstrom, Trifiro and Polgreen reported no associations. Nonetheless, the use of azithromycin now comes with a caution from the FDA of QT prolongation that can lead to torsades de pointes (TdP) and sudden cardiac death (SCD). Adverse cardiac events with azithromycin use are even more pronounced in those with underlying cardiac issues.
In a similar vein, levofloxacin is a fluoroquinolone antibiotic that is also known to prolong the QT-interval (Gorelik et al, Drug Saf 2019). This same group of authors reported a higher short term risk of SCD with levofloxacin use (Assimon et al, JAMA 2022) in those receiving haemodialysis as compared to an amoxicillin-based antibiotic regimen.
The #NephJC session on July 12 and 13 will look at the risk of SCD when patients on dialysis use azithromycin. We will look at the study by Assimon et al, which consists of two retrospective cohort studies, examining cardiac safety of azithromycin vs amoxicillin, and separately, azithromycin and levofloxacin both in the haemodialysis population.
The Study
Population & design
The study population data was taken from the United States Renal Data System (USRDS). Two retrospective cohort studies were done using an active comparator, new-user design (Figure 1) to investigate the comparative risk of SCD in patients (>18 years old) receiving in-center haemodialysis with:
Azithromycin vs amoxicillin-based antibiotic (amoxicillin and/or amoxicllin/clavuronic acid)
Azithromycin vs levofloxacin
Figure 1: Study design. A single antibiotic treatment episode was first identified. In each cohort, the index date was defined as the date of study antibiotic initiation after a 30-day washout period free of relevant antibiotic use. In each cohort, baseline covariates were obtained in the 180-day period prior to the index date.
Antibiotic prescription length
All prescription fills between 1/1/2007 and 12/30/2017 were included in both cohorts. Individual patients could contribute multiple study antibiotic treatment episodes to the analyses.
General exclusion criteria
Receipt of maintenance dialysis for ≤90 days at the start of the baseline period
Lack of continuous Medicare Part A, B, and D coverage during the baseline period
Receipt of hospice care during the baseline period
Presence of an implantable cardioverter defibrillator
Drug prescription exclusion criteria
Azithromycin prescriptions with a prescribed supply >5 days in both cohorts
Amoxicillin-based antibiotic prescriptions with a prescribed supply >10 days in the azithromycin vs. amoxicillin-based antibiotic cohort
Levofloxacin prescriptions with a prescribed days supply >10 days in the azithromycin vs. levofloxacin cohort.
Episodes in which the associated washout period overlapped a preceding, already included treatment episode
Treatment episodes of patients who were hospitalized or residing in a skilled nursing facility at any point during the 30-day washout period (as an outpatient prescription fill following hospitalization/staying at a nursing facility may represent continuation of antibiotic therapy)
Exposure
Outpatient study antibiotic treatment episodes of azithromycin, amoxicillin, amoxicillin/clavulanic acid and levofloxacin
Outcomes
Primary outcome: Sudden cardiac death (SCD)
Secondary outcomes:
A composite of SCD or hospitalized ventricular arrhythmia
Cardiovascular mortality
Outcome period:
Days 1 - 5, capturing active use of both azithromycin and comparator antibiotic
Days 6 -10, capturing active use of comparator antibiotics only
In companion analyses, hospitalized hip fracture was used as a negative control outcome.
Table S1 gives further details on how the authors classified their outcomes.
Covariates
Patient demographics
Comorbid conditions
Prescription medication use
Metrics of health care utilization
Statistical Analysis
Intention-to-treat analytic approach to evaluate the association between azithromycin and comparator antibiotic was used to evaluate the risk of SCD during days 1 - 5 and 6 - 10, separately. Individuals were followed forward in time during the two outcome periods until occurrence of either a:
Study outcome
Censoring event, which are:
Dialysis modality change
Kidney transplantation
Kidney function recovery
Loss of Medicare coverage
Loss to follow up
Completion of 10-days follow-up
Study end (12/31/2017)
Competing event, e.g. death due to cause other than SCD
Primary analyses
Both relative and absolute effect measures to assess study antibiotic-SCD associations were estimated. Hazard ratios (HR) and their 95% confidence intervals (CIs) were estimated using Fine and Gray proportional subdistribution hazard models, wth robust variance estimation to account for within person correlation.
The cumulative incidence of the primary outcome SCD was estimated using the Aalen-Johansen nonparametric estimator.
A risk difference (RD) was then calculated with the formula:
RD = CIt - CIc
Where CIt is the cumulative incidence of the outcome of interest in treatment group and CIc is the cumulative incidence of the outcome in the comparator group
Hence, the RD for SCD = [cumulative incidence of SCD in the azithromycin group] - [cumulative incidence of SCD in the comparator antibiotic group]
95% CIs for RDs were then calculated using a cluster-based bootstrap procedure with 250 resamples to account for within-person correlation of repeated measures.
Propensity matching was used to balance the risk of SCD between groups.
Secondary analyses
In cardiovascular mortality analyses, non-cardiovascular death was treated as a competing event. The risk of SCD in association with higher and lower dose levofloxacin treatment vs azithromycin treatment was also estimated. As these analyses considered three different treatments (i.e., higher dose levofloxacin, lower dose levofloxacin, and azithromycin), propensity scores were estimated using multinomial logistic regression and IPT weights using generated standard methods for multi-categorical exposures.
In companion analyses, the association between azithromycin vs. comparator antibiotic treatment and hospitalized hip fracture, a negative control outcome were done. In these analyses, all-cause death was a competing event.
Post-hoc analyses
The authors examined the gradation of risk across all three antibiotic classes by constructing an additional new-user cohort comprised of azithromycin, levofloxacin, and amoxicillin-based antibiotic treatment episodes. The comparative cardiac safety of azithromycin and levofloxacin vs. amoxicillin-based antibiotics in the three-drug cohort during days 1-5 (a time period that captures active use of all study medications based on typical prescription durations) was then compared using analogous methods to the two two-drug cohorts.
Funding: Several authors have funding from NHLBI/NIH, and have other industry disclosures, which do not seem relevant directly to the study.
Results
Azithromycin vs. Amoxicillin-based Antibiotics
A total of 725,431 antibiotic treatment episodes among 282,899 unique adults were included: 381,306 (52.6%) azithromycin treatment episodes and 344,125 (47.4%) amoxicillin-based antibiotic treatment episodes. Figure S1 shows how the authors arrived at this number.
Figure S1. Flow diagram for the azithromycin vs. amoxicillin-based antibiotic cohort construction
Table 1 shows the baseline patient characteristics of antibiotic treatment episodes in the two study cohorts after inverse probability of treatment weighting.
Table 1: Baseline patient characteristics in both study cohorts
†: Sample sizes of the treatment groups in the pseudo-populations generated after inverse probability of treatment weighting.
‡: QT-prolonging medications of interest included drugs classified as having a known, conditional, or possible risk of torsade de pointes according to the CredibleMeds website (https://crediblemeds.org).
A total of 359 SCDs occurred at a rate of 9.9 events per 100,000 person-days during days 1-5 and a total of 346 SCDs occurred at a rate of 9.6 events per 100,000 person-days during days 6-10.
Compared with amoxicillin-based antibiotic treatment, azithromycin treatment was associated with a higher risk of SCD during days 1-5 (Figure 2, Table 2).
The number needed to harm suggests that 1 additional SCD would occur during the first 5 days of treatment for every 4,000 treatment episodes with azithromycin use vs. an amoxicillin-based antibiotic.
The association was less potent during days 6-10 and secondary analyses considering alternative cardiac outcomes produced consistent results (Table 2).
Figure 2: Azithromycin vs. comparator antibiotic treatment and sudden cardiac death
Table 2: Azithromycin vs. amoxicillin-based antibiotic treatment and cardiac outcomes
Azithromycin vs. Levofloxacin
A total of 554,557 antibiotic treatment episodes among 245,143 unique adults were included —387,382 (69.9%) azithromycin treatment episodes and 167,175 (30.1%) levofloxacin treatment episodes (Figure S3).
A total of 422 SCDs occurred at a rate of 15.2 events per 100,000 person-days during days 1-5 and 349 SCDs at a rate of 12.7 events per 100,000 person-days occurred during days 6-10 (Figure 2, Table 3).
Compared with levofloxacin treatment, azithromycin treatment was associated with a lower risk of SCD during days 1-5 (Table 3). These findings suggest that 1 less SCD would occur during the first 5 days of treatment for every 5,921 treatment episodes with azithromycin use instead of levofloxacin. The association was similar during days 6-10.
Table 3: Azithromycin vs. levofloxacin treatment and cardiac outcomes
Higher and lower doses of levofloxacin vs azithromycin
Both higher and lower dose levofloxacin were associated with higher risk of SCD during days 1-5 and days 6-10 compared to azithromycin treatment (Figure 3). However, the association was more pronounced with higher levofloxacin doses, especially during days 6-10.
Figure 3. Higher and lower dose levofloxacin vs. azithromycin and sudden cardiac death
Negative control outcome analyses
Negative control outcome analyses where the outcome of hospitalized hip fracture was used in evaluating the association between azithromycin and comparator antibiotic treatment produced null results in both new-user cohorts.
Post-hoc analyses: azithromycin and levofloxacin vs. amoxicillin-based antibiotics
Compared to amoxicillin-based antibiotic treatment, levofloxacin treatment was associated with the highest risk of SCD during days 1-5 (Figure 4). SCD risk was elevated during azithromycin treatment, but was lower than the risk associated with levofloxacin treatment.
Figure 4: Levofloxacin and azithromycin vs. amoxicillin-based antibiotics and sudden cardiac death during days 1-5.
Discussion
In this retrospective study, Assimon and colleagues find an elevated risk of sudden cardiac death and cardiovascular mortality in patients with kidney failure on haemodialysis associated with the use of azithromycin compared to amoxicillin-based antibiotics, and levofloxacin compared to azithromycin. Azithromycin is frequently prescribed antimicrobial agent due to its favorable drug interaction profile and convenience of dosing. Azithromycin and levofloxacin are preferred first line agents for many indications. To understand how big the risk is and whether the findings of this study would impact routine clinical practice, it’s essential to evaluate both sides of the argument.
The issue of drug-induced arrhythmias was first recognized when Selzer and Wray documented polymorphic ventricular tachycardia as a cause of Quinidine syncope (Selzer et al, Circulation 1964). Subsequently, several classes of drugs, including antibiotics, have been recognized to precipitate arrhythmia. One mechanism underlying the arrhythmogenicity is the prolongation of myocardial cell action potential (prolongation of QTc in the ECG) by blocking the myocardial cell channels responsible for the potassium outflow current. Azithromycin and levofloxacin are known to be low potency myocardial cell potassium channel blockers (Abo-Salem et al, Cardiovasc Ther 2014), and significant cardiac risk cannot usually be attributed solely to their use if other risk factors are absent. The common risk factors for torsades de pointes (TdP) are structural heart diseases, hypomagnesemia, hypokalemia, and concomitant administration of QTc prolonging drugs. Haemodialysis patients are a special population with a heightened risk for TdP due to decreased drug clearance, electrolyte fluctuations, and multiple unique factors related to the haemodialysis therapy. One such factor is polypharmacy, Assimon previously (Assimon, 2020) showed that use of QT prolonging medications was ~1.4-2.5 times higher in patients on haemodialysis compared to peers not on dialysis.
Strengths
The large cohort size (essential when considering a rare adverse effect)
Stringent exclusion criteria to identify new antibiotic treatment episodes. The investigators used a 30 day washout period, to establish as clean a cohort as possible. In addition, they did control for available covariates using propensity score matching.
Limitations
The pro-arrhythmic potential of a prescribed antibiotic is directly related to the dose (Kannankeril et al, Pharmacol Rev. 2010). Though the investigators identified new treatment episodes through the drug prescription claims, the adherence to the prescribed antibiotic was not assessed, which is more likely to affect the thrice daily amoxicillin arm rather than daily azithromycin.
Other factors unique to haemodialysis like the presence of serum electrolyte abnormalities, use of low calcium/potassium baths, rapid ultrafiltration rates, and intradialytic hypotension (Makar et al, AJKD 2018) were not evaluated, given the limitations of registry data. However there is no reason to expect a difference between the antibiotic arms.
The baseline covariates included a recent ECG done during the last 30 days of baseline. However, it is not known whether an ECG was done prior to the initiation of the antibiotic and subsequently repeated during the treatment course considering the fact that more than 50% of the patients were using one or more medications that elevate TdP risk. Could this have impacted the antibiotic decisions?
The primary outcome in this study was sudden cardiac death as defined by the USRDS definition. The causes of death were obtained from the ESKD death notification forms rather than from the medical records of the patient. Hence, there exists a possibility of misclassification of the outcomes - however this would be expected to be random, and if at all, would potentially bias the results towards the null in most cases. As an aside, sudden cardiac death constitutes a large proportion of the out-of-hospital deaths, but authors do not describe how the out-of-hospital deaths were captured.
Lastly, we cannot talk about the risk without mentioning the benefits of antibiotics. From this study we don’t know the relative rates of other bad outcomes to help us inform our decisions, such as treatment failure or C. Diff. infection when we use antibiotics other than azithromycin or levofloxacin to decrease SCD risk.
This study shows that 1 additional sudden cardiac death would occur during the first 5 days of therapy for every 4,000 treatment episodes when azithromycin is prescribed over amoxicillin-based antibiotics. The study also found that 1 additional sudden cardiac death would occur during the first 5 days of therapy for every 5,291 treatment episodes when levofloxacin is prescribed over azithromycin. Lest one be wary of using NNT (NNH in this case) - this is possibly a scenario when it's somewhat acceptable to use NNH (see Manasi’s #NephStats blog). The time horizon is short enough - though the interpretation remains difficult.
So how worried should we be about a 1 in 4 - 5000 risk? For a better perspective, we can compare this estimate with the risk of death in other iatrogenic complications. The risk of death from drug induced fulminant hepatitis (Sgro et al. Hepatology,2002) or penicillin use related anaphylaxis (Neugut et al. Arch Int Med,2001) is around 1:50,000. This should be weighed against the benefit of using the antibiotic concerned (azithromycin/levofloxacin) versus amoxicillin.
This study definitely raises concerns on the safety of azithromycin and levofloxacin use in haemodialysis patients. It highlights the importance of assessing for various pro-arrhythmic triggers in patients on haemodialysis prior to initiation of these antibiotics. For developing countries, the concerns are much higher considering the prevalence of over-the-counter-sale of these antibiotics. Apart from azithromycin and levofloxacin there are other macrolides and fluoroquinolones whose cardiotoxic potential in the haemodialysis population is not known. But extrapolation from the population without kidney failure would indicate that the risk from moxifloxacin may be higher still.
ECG screening and monitoring are recommended for hospitalized patients receiving QTc-prolonging medications (Drew et al, Circulation 2019). However, no such recommendation exists for ‘non-cardiac’ medications prescribed in outpatient settings. But since the haemodialysis population has a constellation of risk factors which significantly increase the risk of TdP and hence SCD, it seems prudent to obtain a baseline ECG prior to starting therapy with drugs known to cause QTc prolongation and monitor the same during therapy. An increase in QTc from pre-drug baseline of 60ms or a QTc interval prolongation>500 ms after initiation of a drug known to cause TdP are indicative of risk for arrhythmia (Drew et al, Circulation 2019).
Conclusion
Levofloxacin is associated with an increased risk of sudden cardiac death in haemodialysis patients compared to azithromycin which in turn was associated with a higher risk of SCD compared to amoxicillin-based antibiotics. Antibiotic choices in haemodialysis patients should not just be based on the antimicrobial profile. They should take into consideration the potential cardiac risk and precipitating factors for sudden cardiac death.
Summary prepared by
Rachel Hung
Nephrology Specialist Registrar, PhD Student
King’s College London, United Kingdom
&
Anand Chellappan
Assistant Professor, Department of Nephrology
All India Institute of Medical Sciences (AIIMS), Nagpur, India
NSMC Interns, Class of 2022
Reviewed by Joel Topf, Shina Menon, Jamie Willows, Jade Teakell, and Swapnil Hiremath